
Agriculture
June 23, 2024
AguaPallet
Read SolutionImplemented by
LoooP Creative Ltd
Updated on February 16, 2024
·Created on July 8, 2016
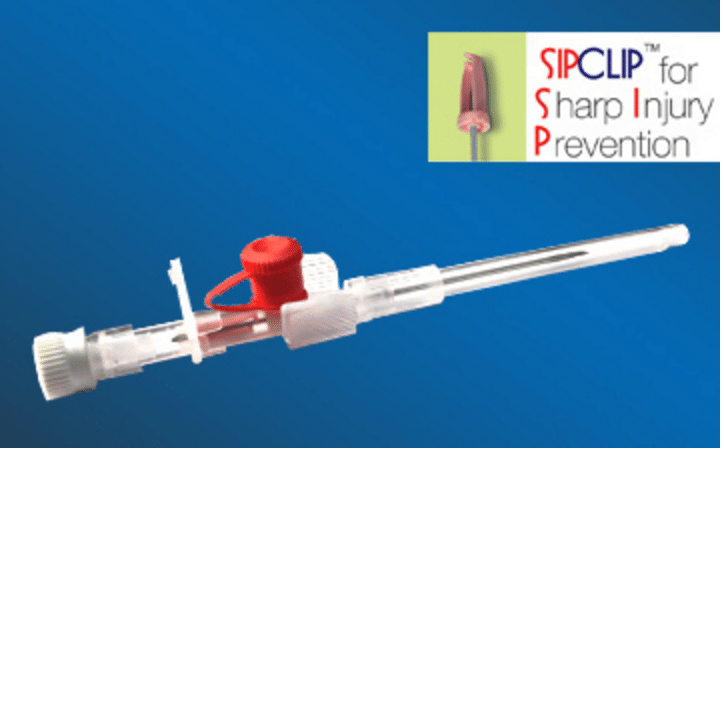
Cathy Sip Clip I.V Cannulas prevents accidental needle stick injury provided that general effective and safe working procedures and precautions are maintained during its use and disposal.
Cathy Sip Clip I.V Cannulas is a cost-effective device to prevent accidental needle stick injuries. It is designed with a safety mechanism activated automatically on withdrawal of needle post cannulation.
Cathy SiP CliP has an innovative plastic clip that automatically covers the blood contaminated needle tip as its removed from the IV Catheter to prevent any needle stick injury to the nurse or Doctor and any risk of infection of patient migrating to them.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$0.02
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Sip Clip has partnered with Hindustan Syringes and Medical Devices Limited (HMD) , India's leading manufacturer of auto-disabling and single-use syringes for manufacturing and distribution of the Cathy Sip Clip I.V Cannulas. The product can be purchased on HMD's website.
Competitive Landscape
Direct competitors include PINNIX Light Safety Blood Lancet.
Regions
Europe, Middle East
Countries
India, United States
Manufacturing/Building Method
Mass-produced. HMD is having technical collaboration with M/s.Vigmed of Sweden to manufacture Safety I.V.Cannula. It is injection molded in one piece.
Intellectural Property Type
Patent
User Provision Model
Users will need to purchase directly from the manufacturer or through the manufacturer HMD's customer base of dealers, which is cited as being over 4000. This is based on Quid ProQuo whereby HMD will be OEM sub-contractor to Vigmed for the European market and Licensee for the Indian and Developing World market and sell under the HMD brand.
Distributions to Date Status
Unknown, but as of 2015 over 1.5million to Scandanavia alone.
Construction material
Needle: Siliconised electropolished, back cut bevelled stainless steel needle Catheter: Available in Volex™ polyurethane radio detectable, a proprietary pure polymer
Disposal Method
Standard sharps disposal methods should be used. The clip mechanism on the device prevents sharps injury.
Reuse prevention method
Unknown
Sterilization method
Unknown
Clinical application
IV Cannulation
Indispensable equipment for function (Y/N)
Yes, medication being administered via cannula
Maintenance or calibration required by user at time of use? (Y/N)
N
Design Specifications
Needle : Siliconised electropolished, back cut bevelled stainless steel needle. * With notch on 24G & 26G Cannula
Sip Clip : Sharps Injury Prevention Clip
Catherter : Available in Volex™ polyurethane radio detectable.
Needle hub : Allows blood flash back visualisation. Thumb rest for proper grip during insertion.
Blood stopper : The stopper has fine air vent to expedite blood flash back for enabling correct cannulation.
Luer lock cap : Closure to wing housing permits patient mobility when infusion temporarily discontinued.
Injection port cap : It facilitates extra medication and also prevents its back flow.
Blister package : I.V. Cannula is packed in rigid blister and sealed in peel open type medical grade paper.
Specifications such as gauge, extension of catheter (diameter and length), and water flow rate are listed on HMD's product page.
Technical Support
This is not applicable. However, the product page states a warning not to use this product if the pack has been previously opened or damaged.
Replacement Components
None, single-use product
Lifecycle
Single use only. Do not use if pack is previously opened or damaged.
Manufacturer Specified Performance Parameters
According to WHO, if safety cannula are made mandatory in hospitals, then around 80 per cent of all hospital staff can be saved from needle stick injuries. HMD sees this as a goal of their product.
Vetted Performance Status
Samples of Cathy +I.V. Cannula were given for clinical trial in leading hospitals and were found to be of satisfactory quality.
Safety
I.V. Cannula is packed in rigid blister and sealed in peel open type medical grade paper.
As the product is designed to guarantee a needle auto-protection safety mechanism, a functional Safety+ IV Cannula should be safe to use. No training should be required to deploy the safety mechanism, though experience with standard IV catheters is likely needed to understand proper usage. Additionally, improper syringe usage can still carry risks to the patient such as infection or embolism.
SIPCLIP prevents accidental needle stick injury provided that general effective and safe working procedures and precautions are maintained during its use and disposal.
Complementary Technical Systems
None
Academic Research and References
Chomnard, P., Jittaporn, C., Anucha, A., Somwang, D., 2005, Effectiveness of Education and Problem Solving Work Group on Nursing Practices to Prevent Needlestick and Sharp Injury. J Med Assoc Thai Vol, 88, pp. S115-S119.
Janine, J., Ella, H., Richard, D., 1990, Sharp object injuries in the hospital: Causes and strategies for prevention, American Journal of Infection Control. 18(4), pp.227-231/
Cathryn, M., 2008, Improved surveillance and mandated use of sharps with engineered sharp injury protections: a national call to action. Healthcare infection. 13(2), pp. 33-37.
Compliance with regulations
The official press release states that these products are manufactured as per ISO 9001, ISO 13485, and the European M.D.D. & US FDA standards.
Evaluation methods
Specific methods unknown, but samples of Cathy +I.V. Cannula were given for clinical trial in leading hospitals and were found to be of satisfactory quality.
Other Information
The flow rate for the catheter ranges between 15-200 ml/min dependent on gauge used. The specifications can be found on this website. For safety, standard sharps disposal methods should be used.

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief

Agriculture
December 31, 2023
Implemented by
Potential Energy

Agriculture
June 22, 2024
Implemented by
World Bicycle Relief

Agriculture
November 19, 2024
Implemented by
Kenya Stove Works Ltd.
Agriculture
March 11, 2024
Implemented by
Capnia

Agriculture
December 18, 2023
Implemented by
Eco Femme

Agriculture
December 3, 2024
Implemented by
Envirofit International
Agriculture
February 5, 2024
Implemented by
EyeJusters

Agriculture
February 8, 2024
Implemented by
GE Healthcare
Have thoughts on how we can improve?
Give Us Feedback