
Agriculture
June 23, 2024
AguaPallet
Read SolutionImplemented by
LoooP Creative Ltd
Updated on September 26, 2024
·Created on February 5, 2022
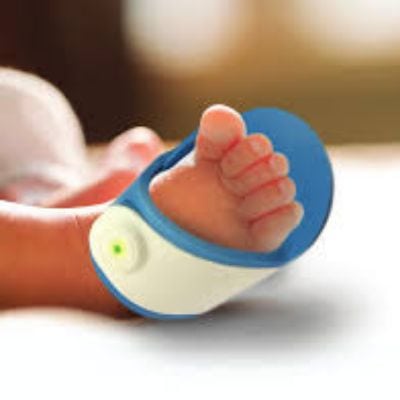
The Bempu ApneBoot is a shoe that monitors, alerts, and treats for infant episodes of central apnea.
The ApneBoot is an apnea monitoring device for infants developed by Bempu Health. The device is in the prototype stage and is currently undergoing clinical validation. ApneBoot is a shoe that monitors an infant for episodes of central apnea. When one is detected, the shoe vibrates the infant’s foot (stimulating the nervous system to restart breathing) to prevent apnea related damage. It sends out alarms (using both sound and light) to alert nurses and caretakers that an episode of central apnea is occurring. The ApenBoot can be used in homes, wards, neonatal intensive care units, and during medical transport.
Target Regions
Central Africa, South America, West Africa
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Household, Community, Small and Medium-sized Enterprises
Distributors / Implementing Organizations
Bempu Health
Competitive Landscape
Direct competitors include neoGuard Vital Signs Monitor for Newborns.
Regions
Central Africa, South America, West Africa
Countries
India
Manufacturing/Building Method
ApneBoot is currently a prototype and manufacturing has not yet begun.
Intellectural Property Type
Select Type
User Provision Model
Bempu Health's first product, the TempWatch, is sold directly through the manufacturer. The ApneBoot is still in development.
Distributions to Date Status
None
Alert mechanism
Sound and LED
Clinical measurements monitored
Blood oxygen saturation and pulse rate
Patient population
Preterm babies and at-risk newborns
Maximum duration of consecutive usage
72 hrs (estimated)
Design Specifications
The ApneBoot monitors the pulse rate and oxygen saturation of the infant using an SpO2 sensor. If an episode of apnea is detected, the shoe vibrates, waking the infant and stimulating the nervous system to restart breathing. It also alerts caretakers if apnea is detected. The ApneBoot is designed with medical grade silicon so it is easy to strap on, easy to clean and disinfect, and is comfortable for the infant. It is also designed to fit babies of varying weight.
Technical Support
Provided by the manufacturer
Replacement Components
Battery
Lifecycle
This product is a prototype so the lifecycle is not yet known.
Manufacturer Specified Performance Parameters
Responds instantaneously
Automatically stimulates the infants foot
No training required
Usable in clinic, home, and transport as well as during Kangaroo Mother Care
Vetted Performance Status
Clinical trials are currently being conducted by Bempu Health. Preliminary findings from a non-randomized study at Niloufer Hospital, Hyderabad found that 44% of apneas resolved within 15 seconds, likely attributed to the ApneBoot. 53% of apneas did not resolve in 15 seconds, most likely because they were not central apneas.
Safety
No known safety hazards are related to this product
Complementary Technical Systems
None
Academic Research and References
None
Compliance with regulations
Unknown

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief
Agriculture
December 4, 2023
Implemented by
Uganda Industrial Research Institute
Agriculture
February 21, 2024
Implemented by
NONSPEC
Agriculture
March 9, 2024
Implemented by
Mirola Rescue

Agriculture
March 8, 2024
Implemented by
Pacific Consolidated Industries

Agriculture
February 22, 2024

Agriculture
January 31, 2024
Implemented by
Amparo GmbH

Agriculture
June 28, 2024
Implemented by
Pipistrel

Agriculture
June 29, 2024
Implemented by
Volansi
Have thoughts on how we can improve?
Give Us Feedback
Overall I think this is a good overview of this product. A few comments include:
1. This states that this product doesn’t use any consumables although a battery is used (which only provides an estimated 72 hours of use). It is unclear if the product is intended to be rechargeable, disposable or have the batteries replaced.
2. If not intended for one-time use, it would be valuable to have an estimate on how many times the unit can be used before needing to be replaced. Even if the actual lifecycle is not known, stating the “as designed” lifecycle would be helpful in better understanding the product. It can even be as simple as selecting an appropriate order of magnitude (1, 10, 100, 1000…).
3. If it is a reusable device, some insight into how the device will need to be cleaned/sanitized would be valuable. Particularly since the target market is the developing world where many methods are unavailable or difficult to come by.
4. There is a typo in the “Design Specifications” section where it reads “pulse rat” and I believe it is intended to be “pulse rate”.