Not too long ago, the health scourges in the developing world were a nearly exclusive club of communicable diseases such as HIV/AIDS, polio, and tuberculosis. As living conditions have improved, the health risks have shifted to more closely resemble those of the developed world. The incidence of non-communicable and chronic diseases such as diabetes, heart disease, and cancer has drastically increased globally. In particular, sedentary lifestyles and changing diets in the developing world have led to skyrocketing type 2 diabetes diagnoses, affecting people from India and South Asia to Africa [1][2].
But the problem could be worse than the diagnosis rate indicates. The International Diabetes Foundation (IDF) estimates that two-thirds of the diabetic population remain undiagnosed. Unfortunately, health systems in resource-limited countries are ill-equipped to deal with this “double disease burden.” Implementing diabetes screening has the potential to identify these undiagnosed individuals earlier in the disease’s progression and reduce harmful complications.
So, how is it possible to screen hundreds of millions of people for diabetes? The earliest screening for diabetes was documented by physicians in India, who noted that ants and bees were attracted to the urine of people with diabetes [3]. Diabetics release more sugars in the form of glucose into their urine, which attracts the insects. Current screening methods rely on the same principle, measuring glucose in blood or urine. And another common screening tool is a risk assessment questionnaire [4].
These methods are widely used in the Western world, but the developing world lacks the resources to analyze blood samples on a large scale, and screening tests are expensive to distribute. Health systems in the developing world rely largely on external funding, and diabetes programs remain underfunded. [5].
Those obstacles present opportunities for social entrepreneurs, however. A number of groups are working on innovative, low-cost methods to screen for diabetes in resource-constrained settings. Here are the top nine approaches currently in development.
Inkjet printing for blood testing
A team of students in the bioengineering department of Clemson University have repurposed an inkjet printer to manufacture low-cost blood glucose test strips. By replacing the ink with specialized enzymes, the team is able to print test strips that change color when glucose is present [6]. The strips cost only 5% of the cost of commercially-available strips.
Inkjet printing and stamping for urinalysis
Our group, the Humanitarian Engineering and Social Entrepreneurship (HESE) program at Penn State University and REACH Diagnostics, a social enterprise started at York University, are applying inkjet printing to make low-cost urinalysis strips as an alternative to blood testing. Other groups are trying a similar tactic. The goal would be to set up local manufacturing centers in hard-to-reach, low-resource areas. Basing them there simplifies the supply chain, making diabetes screening more accessible [7]
Our Penn State HESE program is also developing a method of stamping the chemical assays onto specialized filter paper for use in areas where printer maintenance would be difficult.
Microfluidic devices

Paper-based microfluidic strips like these liver-function tests can also screen for diabetes. Photo courtesy of Diagnostics for All
The Whitesides lab at Harvard University has developed an inexpensive microfluidic device, called a μPAD, that can be used to screen for a wide variety of relevant diseases [8]. Like test strips, this paper-based system changes colors in the presence of certain biomarkers such as glucose. The team is working on an integrated telemedicine system that would allow the color changes to be analyzed by a doctor regardless of location.
Breath measurements
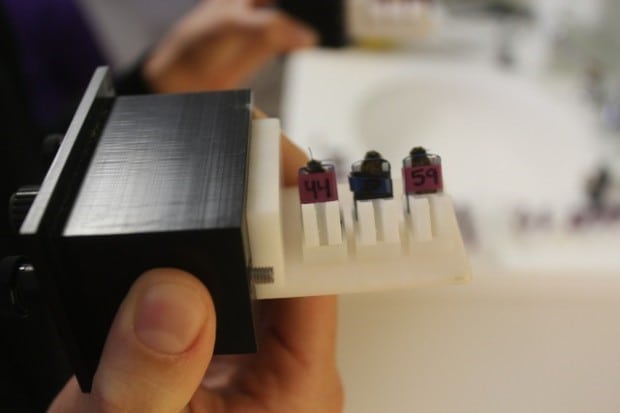
Bee Healthy is developing this diabetes screening device that uses bees. Photo courtesy of commonhealth.wbur.org
Many of the volatile organic compounds (VOCs) present in human breath could be harnessed for screening or diagnostic purposes [9]. In particular, acetone is a compound that when released during exhalation is a marker for diabetes [10]. Several groups have developed preliminary biosensors that can detect acetone in a breath [11], [12]. These devices have yet to be used in clinical settings to detect diabetes, so their sensitivity and specificity as screening devices remains unknown.
Chemical biosensors are not the only option for detecting acetone in breath. Bee Healthy, a social enterprise and 2014 Hult Prize finalist, is attempting to train bees to react to specific concentrations of acetone in an air sample [13]. Bees are tens of thousands of times more sensitive to chemical signals in the air than humans and are easily trained through classical conditioning. The Bee Healthy team has successfully used the bees to identify diabetics with a 70% success rate. While the sensitivity of this method could be improved, using bees to screen for diabetes has promise.
Nanoparticle-based sensor
A team of researchers from the University of Cambridge has used nanotechnology to make a low-cost and reusable glucose sensor [14]. Silver nanoparticles embedded in a hydrogel exhibit a wavelength shift, and therefore a visible color change, when glucose is present in a urine sample. The sensor can be used hundreds of times without compromising accuracy, though the complex manufacturing process requires specialized training and equipment.
Handgrip Strength
A team in the Penn State HESE program has investigated the feasibility of using handgrip strength (HGS) in conjunction with a simple risk factor assessment to screen individuals for diabetes in the developing world [15]. Inexpensive yet durable hand dynamometers are currently manufactured by several companies, which make HGS a viable metric to collect in resource-constrained settings. Current screening algorithms that use HGS need to be refined to improve the sensitivity and specificity of this method.
Skin Biomarkers
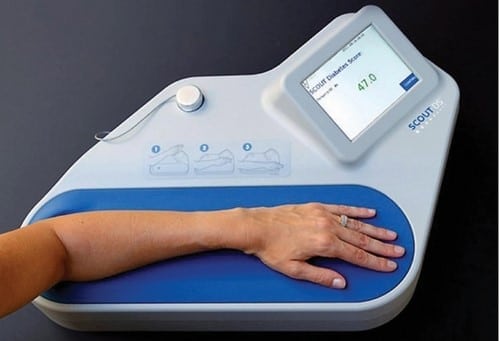
Photo courtesy of the Scout DS
Medical device company Miraculins is in the process of commercializing the Scout DS®, a diabetes screening device that uses light to detect specific biomarkers in the skin [16]. Because this method is no more harmful than shining a flashlight at a patient’s arm, it has potential to avoid the risks, such as infection, inherent to invasive procedures. However, the device will need to undergo modifications to decrease the cost before it can be adopted in the developing world.
Fingernail clippings
A team of researchers from Ghent University in Belgium is investigating the use of fingernail clippings as an avenue of screening for diabetes noninvasively [17]. The group observed that the fingernails of patients with diabetes had significantly higher levels of a specific type of diabetes marker. Using fingernail clippings instead of blood avoids issues of safety and storage that complicate blood and urine based screening methods.
All of these methods are promising alternatives that have the potential to be better-suited to the developing world than traditional blood glucose tests.
The foundation of a health program: Community Health Workers
One of the largest challenges to implementing wide-scale diabetes screening programs is reaching rural and underserved populations, who may live far from the nearest clinic or hospital. Many countries have established Community Health Worker (CHW) programs to improve access to care for these populations. CHW networks may be leveraged to deliver diabetes screening to people in rural areas. In addition, CHWs (most of whom work on a volunteer basis), may earn an income selling diabetes screening tests to community members. A financial incentive would not only provide motivation to conduct diabetes screenings but give CHWs more flexibility to continue their other healthcare duties. In addition, demonstrating the financial viability of health screening services could have a larger positive effect on the community. Mashavu, a social enterprise that trains CHWs to provide basic health screenings in exchange for a small fee, was launched in 2009 in Nyeri, Kenya, by students at Penn State. Since then, over 30 local businesses have been launched that provide some sort of basic health service, such as blood pressure measurements.
While screening for diabetes is just the first step in improving how diabetes is identified and managed in the developing world, it is an important way to increase awareness of the disease and motivate individuals to seek further care.
References
[1] P. Shetty, “Public health: India’s diabetes time bomb.,” Nature, vol. 485, no. 7398, pp. S14–6, May 2012.
[2] V. Hall, R. W. Thomsen, O. Henriksen, and N. Lohse, “Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review.,” BMC Public Health, vol. 11, no. 1, p. 564, Jan. 2011.
[3] Principles of Diabetes Mellitus, vol. 1. Springer Science & Business Media, 2010.
[4] World Health Organization, “Screening for type 2 diabetes,” World Health Organization, 2003.
[5] D. Pustay, K. Mehta, and P. Munyua, “Strategies to support non-communicable disease interventions,” Int. J. Soc. Entrep. Innov., Oct. 2015.
[6] K. Gainey Wilson, P. Ovington, and D. Dean, “A Low-Cost Inkjet-Printed Glucose Test Strip System for Resource-Poor Settings.,” J. Diabetes Sci. Technol., vol. 9, no. 6, pp. 1275–81, Nov. 2015.
[7] A. Ranslow, D. Crompton, K. Mehta, P. Butler, and J. Adair, “Empowering Community Health Workers with Inkjet-printed Diagnostic Test Strips,” Procedia Eng., vol. 107, pp. 205–214, 2015.
[8] A. W. Martinez, S. T. Phillips, G. M. Whitesides, and E. Carrilho, “Diagnostics for the developing world: microfluidic paper-based analytical devices.,” Anal. Chem., vol. 82, no. 1, pp. 3–10, Jan. 2010.
[9] A. Riley, S. Krisher, and K. Mehta, “Breath and Air Analysis: Applications in Resource-poor Settings,” Procedia Eng., vol. 107, pp. 215–222, 2015.
[10] Z. Wang and C. Wang, “Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements.,” J. Breath Res., vol. 7, no. 3, p. 037109, Sep. 2013.
[11] M. Ye, P.-J. Chien, K. Toma, T. Arakawa, and K. Mitsubayashi, “An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath.,” Biosens. Bioelectron., vol. 73, pp. 208–13, Nov. 2015.
[12] T. I. Nasution, I. Nainggolan, S. D. Hutagalung, K. R. Ahmad, and Z. A. Ahmad, “The sensing mechanism and detection of low concentration acetone using chitosan-based sensors,” Sensors Actuators B Chem., vol. 177, pp. 522–528, Feb. 2013.
[13] “Let’s Explore Diabetes With Honeybees (Seriously — It Could Work In Urban Slums) | CommonHealth.” [Online]. Available: http://commonhealth.wbur.org/2014/08/lets-explore-diabetes-with-honeybees. [Accessed: 21-Mar-2016].
[14] A. K. Yetisen, Y. Montelongo, F. da Cruz Vasconcellos, J. L. Martinez-Hurtado, S. Neupane, H. Butt, M. M. Qasim, J. Blyth, K. Burling, J. B. Carmody, M. Evans, T. D. Wilkinson, L. T. Kubota, M. J. Monteiro, and C. R. Lowe, “Reusable, robust, and accurate laser-generated photonic nanosensor.,” Nano Lett., vol. 14, no. 6, pp. 3587–93, Jun. 2014.
[15] M. Eckman, C. Gigliotti, S. Sutermaster, and K. Mehta, “Get a grip! Handgrip strength as a health screening tool,” in IEEE Global Humanitarian Technology Conference (GHTC 2014), 2014, pp. 242–248.
[16] “Miraculins – Diabetes Program.” [Online]. Available: http://www.miraculins.com/diabetes_program.aspx. [Accessed: 05-Apr-2016].
[17] A. S. Kishabongo, P. Katchunga, E. H. Van Aken, M. M. Speeckaert, S. Lagniau, D. Husein, Y. E. Taes, and J. R. Delanghe, “Glycated nail proteins: a new approach for detecting diabetes in developing countries.,” Trop. Med. Int. Health, vol. 19, no. 1, pp. 58–64, Jan. 2014.


Dear Sir
I am sridhar working for PROMOTION of micro ENTERPRISES to urban women poor in andhra pradesh .please advise if any livelihood ACTIVITIES can be setup at urban levels in andhra pradesh. i would like to introduce health kisok at each sulm .please let me know to go further
thanking you
sridhar
9989334060