Agriculture
February 8, 2024
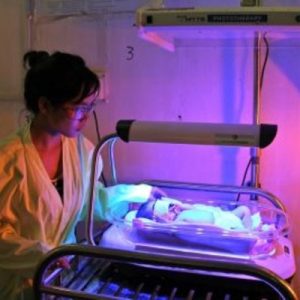
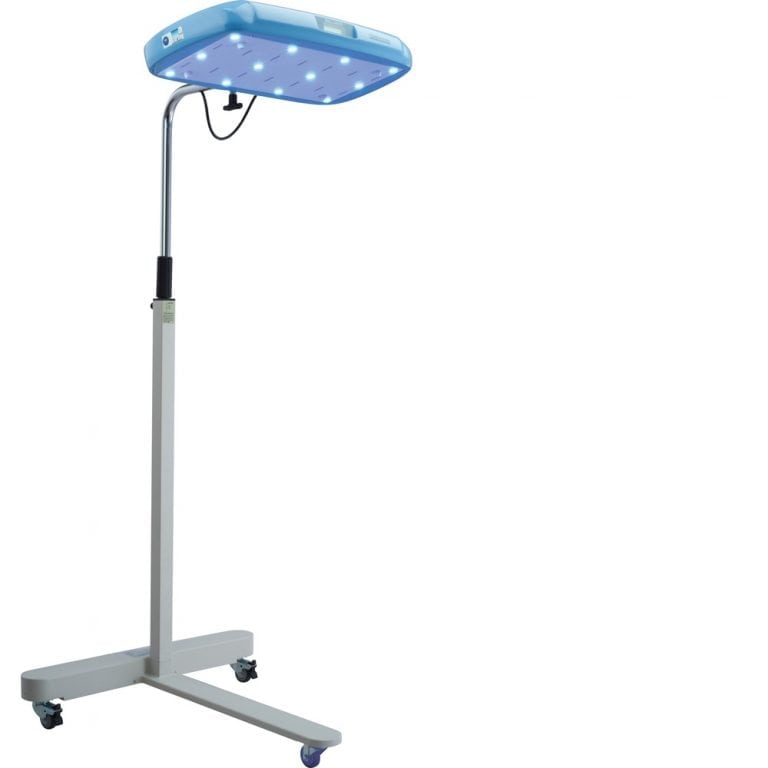
Firefly Phototherapy Device
Read SolutionImplemented by
Design That Matters (DtM)
Updated on February 21, 2024
·Created on June 4, 2014
Brilliant Phototherapy Device is a neonatal jaundice treatment device.
Brilliance Phototherapy Device is a blue LED-based phototherapy device for treatment of neonatal jaundice.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$400.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Phoenix Medical Systems Private Ltd in Chennai India manufactures, distributes, and sells Brilliance under a license from D-Rev
Competitive Landscape
Direct competitors include Little Sparrows Technologies Bili-Hut and Firefly Phototherapy Device.
Regions
Africa, Asia, Europe, Oceania, South America
Manufacturing/Building Method
Manufactured in India
Intellectural Property Type
Patent
User Provision Model
For sale globally through Phoenix Medical Systems. Users can submit an enquiry form or contact the manufacturer through the phone.
Distributions to Date Status
As of 2015, 1,204 units have been installed in 15 countries. Interview with designer, May 2015
As of 2020, over 960,000 babies have been treated with Brilliance Phototherapy Device in 61 countries.
Average spectral irradiance (μW/cm2/nm)
Average shown via irradiance map.
Peak > 40 µW/cm²/nm at 35 cm treatment distance (measured with Ohmeda Medical BiliBlanket II Meter)
Device dimensions
159.5 cm x 71 cm x 71 cm
Device weight (kg)
20
Effective surface area (cm)
25 x 50 (at recommended treatment distance)
Illumination source
Blue LEDs
Irradiance uniformity ratio
Min/Max irradiance > 0.4
Lamp duration (hrs)
50,000+ (designer spec of 9.8 years, assuming 18 hrs usage per day)
Max power consumption (W)
60
Peak wavelength (nm)
450 – 465
Recommended treatment distance (cm)
35
Time totalizer (machine run/therapy)
Machine run, therapy time
Voltage type
100 – 240 VAC
Design Specifications
* Overall Dimensions: 159.5 cm (height) x 71 cm (width) x 71 cm (depth)
* Light Unit Dimensions: 57.5 cm (length) x 36 cm (width) x 6 cm (depth)
* Total Unit Mass: 20 kg
* Footprint of Stand: 71 cm (length) x 71 cm (width) x 13 cm (depth)
* Height of Base <7.5 cm
* Castors: 2 Lockable
* Height Adjustment Range: 110 cm-160 cm (distance between bottom surface and ground)
* Recommended treatment distance: 35 cm
* Electrical supply: voltage 100-240 VAC ~; frequency 50/60 Hz
* Max power consumption: 60 W
Technical Support
Several hours of training are required for physicians, nurses, or midwives who will be responsible for placing infant under the device and overseeing their care.
Replacement Components
Brilliance LEDs last up to 120,000 hours and will not need to be replaced for the life of the device. Use of LEDs has minimized maintenance requirements as they require replacement every 10 years compared to other common phototherapy devices that use compact fluorescent light bulbs that require replacement every 3-5 months.
Lifecycle
Product has a lifetime of 10 years with proper care and maintenance (assuming 18 hrs usage per day). Warranty information available from Phoenix Medical Systems.
Manufacturer Specified Performance Parameters
Manufacturer specified performance targets include: able to operate in areas of unreliable power supply, low cost, easy to use, portable.
Vetted Performance Status
During tests at Stanford University School of Medicine, Brilliance outperformed state-of-the-art phototherapy devices (that often cost upwards of $3,000 USD). Brilliance was also found to meet American Academy of Pediatrics standards for effective jaundice treatment Brilliance received CE mark in October 2013, certifying that the product meets European Union standards for safety and quality.
Safety
Error in obtaining the proper distance between the patient and the phototherapy device can result in under or over dose to the patient. The device has a built-in over temperature cut off to maintain safe temperatures.
Complementary Technical Systems
Eye covers, Gonad covers, Bassinet
Academic Research and References
V. K. Cline et. al., Evaluation of an Affordable LED Phototherapy Device for Resource-Limited Settings, in 2011 Pediatric Academic Societies (PAS) and Asian Society for Pediatric Research (ASPR), 2011.
M. C. Juarez and A. L. Grossberg, “Phototherapy in the Pediatric Population,” Dermatologic Clinics, vol. 38, no. 1. W.B. Saunders, pp. 91–108, 01-Jan-2020, doi: 10.1016/j.det.2019.08.012.
R. Pugashetti and J. Koo, Phototherapy in Pediatric Patients: Choosing the Appropriate Treatment Option, YSDER, vol. 29, pp. 115–120, 2010, doi: 10.1016/j.sder.2010.03.006.
A. Paši? et al., Phototherapy in pediatric patients, Pediatr. Dermatol., vol. 20, no. 1, pp. 71–77, Jan. 2003, doi: 10.1046/j.1525-1470.2003.03016.x.
Compliance with regulations
IEC 60601, ISO 14971, ISO 15223-1, ISO 9001:2000, ISO 13485:2003
Evaluation methods
Stanford University study used the Standardized Bench Method (as referenced by: VremanHJ, Wong RJ, Murdock JR, Stevenson DK. Standardized bench method for evaluating the efficacy of phototherapy devices. Acta Paediatr 2008; 97: 308-316.)
Other Information

Agriculture
February 8, 2024
Implemented by
Design That Matters (DtM)

Agriculture
February 16, 2024
Implemented by
Equalize Health

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab
Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360

Agriculture
November 22, 2023
Implemented by
The NeoRest team at the Seattle Children’s Hopsital

Agriculture
February 29, 2024
Implemented by
Jian Zhong Shang
Agriculture
December 12, 2023
Implemented by
Sohum Innovation lab
Agriculture
December 19, 2023
Implemented by
Star Syringe

Agriculture
September 26, 2024
Implemented by
Jorge Odón

Agriculture
February 26, 2024
Implemented by
Hemex Health

Agriculture
February 8, 2024
Implemented by
Design That Matters (DtM)

Agriculture
February 16, 2024
Implemented by
Equalize Health

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360

Agriculture
November 22, 2023
Implemented by
The NeoRest team at the Seattle Children’s Hopsital

Agriculture
February 29, 2024
Implemented by
Jian Zhong Shang

Agriculture
December 12, 2023
Implemented by
Sohum Innovation lab

Agriculture
December 19, 2023
Implemented by
Star Syringe

Agriculture
September 26, 2024
Implemented by
Jorge Odón

Agriculture
February 26, 2024
Implemented by
Hemex Health

Agriculture
February 8, 2024
Implemented by
Design That Matters (DtM)

Agriculture
February 16, 2024
Implemented by
Equalize Health

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360

Agriculture
November 22, 2023
Implemented by
The NeoRest team at the Seattle Children’s Hopsital

Agriculture
February 29, 2024
Implemented by
Jian Zhong Shang

Agriculture
December 12, 2023
Implemented by
Sohum Innovation lab

Agriculture
December 19, 2023
Implemented by
Star Syringe

Agriculture
September 26, 2024
Implemented by
Jorge Odón

Agriculture
February 26, 2024
Implemented by
Hemex Health
Have thoughts on how we can improve?
Give Us FeedbackNotifications