
Agriculture
February 5, 2024
DripAssist Infusion Rate Monitor
Read SolutionImplemented by
Shift Labs

Updated on March 11, 2024
·Created on August 7, 2017
The CoSense® ETCO Monitor uses Sensalyze™ Technology which automates non-invasive detection of infant’s blood pressure through the breath.
The CoSense® ETCO Monitor uses Sensalyze™ Technology which uses a small tube to collect the breath sample to be fed into the monitor to project the reading. The device works with normal breathing and takes 30 seconds to record. The monitor displays the results in 3-4 minutes.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
Capnia, Inc. and Healthware Private Limited (in India)

Agriculture
February 5, 2024
Implemented by
Shift Labs
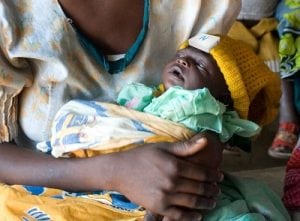
Agriculture
September 27, 2024
Implemented by
Neopenda
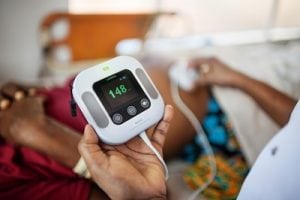
Agriculture
September 26, 2024
Implemented by
Laerdal Global Health

Agriculture
January 11, 2024
Implemented by
Kenya Ceramic Project (KCP)

Agriculture
November 30, 2024
Implemented by
El Fuego del Sol
Agriculture
February 8, 2024
Implemented by
Design That Matters (DtM)

Agriculture
January 10, 2024
Implemented by
NativeEnergy
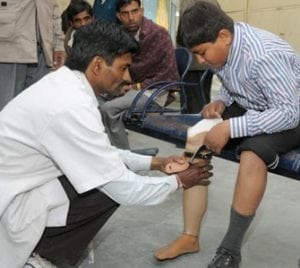
Agriculture
December 1, 2023
Implemented by
SMS Medical College Hospital (Ram Chandra Sharma with the team of doctors)

Agriculture
January 2, 2024
Implemented by
Klorfasil

Agriculture
December 29, 2023
Implemented by
RealRelief
Have thoughts on how we can improve?
Give Us Feedback