Agriculture
February 21, 2024
Brilliance Phototherapy Device
Read SolutionImplemented by
Equalize Health

Updated on February 16, 2024
·Created on May 26, 2016
A phototherapy device to treat neonatal jaundice
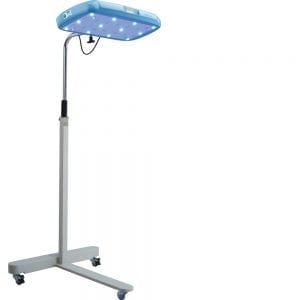
D-Rev’s Brilliance Pro is a blue LED-based phototherapy device for treatment of neonatal jaundice with an integrated light meter, the latest model in their line of phototherapy devices.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$500.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Phoenix Medical Systems Private Ltd in Chennai India manufactures, distributes, and sells Brilliance under a license from D-Rev. “D-Rev listens to customers and works with Phoenix so the product and manufacturing capabilities are aligned; it is a true partnership. The cooperation will create more products at affordable costs, and be a catalyst for market-change,” said Sashi Kumar, managing director of Phoenix Medical Systems.
Competitive Landscape
Direct competitors include Little Sparrows Technologies Bili-Hut, Bilisense Jaundice Treatment Pad, and Firefly Phototherapy Device.
Countries
Colombia, Ecuador, Guyana, India, Kenya, Malawi, Malaysia, Nepal, Nigeria, Pakistan, Philippines, South Africa, Tanzania, Thailand, Uganda
Manufacturing/Building Method
Mass produced
Intellectural Property Type
Patent
User Provision Model
For sale globally through Phoenix Medical Systems.
Distributions to Date Status
With 1,100 units of predecessor Brilliance Classic installed in 23 countries, D-Rev's neonatal jaundice initiative product manager AJ Viola expects to sell around 800 units of Brilliance Classic and Brilliance Pro within the first year of Brilliance Pro's release, with the split between the two still uncertain.
Average spectral irradiance (μW/cm2/nm)
Uniform LED Intensity cited as >45µW/cm²/nm in product specifications sheet.
Device dimensions
As described in specifications sheet: Overall Dimensions Height x Width x Depth: 165cm x 69cm x 64cm Light Unit Dimensions Length x Width x Depth: 53cm x 30cm x 5cm
Device weight (kg)
20 kg
Effective surface area (cm)
>1300cm² at recommended treatment distance
Illumination source
Blue LEDs and White observation lights
Irradiance uniformity ratio
>0.4 (minimum/maximum irradiance)
Lamp duration (hrs)
LED lifetime of 120,000 hours, which is cited as 60x longer than compact fluorescent lamps commonly used in phototherapy devices.
Max power consumption (W)
25W
Peak wavelength (nm)
450-465 nm
Recommended treatment distance (cm)
35cm
Time totalizer (machine run/therapy)
Machine run time totalizer + Therapy time totalizer
Voltage type
Medical-grade Electrical Supply Voltage of 100-240 VAC ~ Frequency of 50/60 Hz
Design Specifications
Specifications
Technical Support
While not explicitly stated on company materials, D-Rev's partnership with Phoenix Medical Systems for manufacturing and distribution suggests that technical support should be available through Phoenix Medical Systems. Phoenix Medical Systems' website includes a section for customer care both in India and internationally, which describes a team that attends to service calls and assists in equipment installation and maintenance operations.
Replacement Components
Use of LEDs has minimized maintenance requirements as they require replacement every 10 years compared to other common phototherapy devices that use compact fluorescent light bulbs that require replacement every 3-5 months . D-Rev argues that, with Brilliance, hospitals can save over $240 USD per year on costly bulb replacements.
Lifecycle
Brilliance LEDs last over 120,000 hours, 60x longer than compact fluorescent lamps commonly used in phototherapy devices.
Manufacturer Specified Performance Parameters
Performance targets specified by D-Rev's manufacturer Phoenix Medical Systems include:
* Illumination source: Blue LEDs + White observation lights
* LED Intensity: > 40 µW/cm²/nm at recommended treatment distance measured with Ohmeda Medical BiliBlanket II Meter
* Peak Wavelength: 451 nm
* Emission Spectrum: 445 – 470 nm (maximum bounds of FWHM range)
* Effective Treatment Area: 25 cm x 50 cm at recommended treatment distance
* Irradiance Ratio: >0.4 (minimum/maximum irradiance)
*LCD: Backlit
* Time Totalizer: Machine run time totalizer + Therapy time totalizer
Vetted Performance Status
Brilliance Pro meets the American Academy of Pediatrics standards for effective jaundice treatment. The American Academy of Pediatrics (AAP) recommends a uniform irradiance of ?30µW/cm2/nm over maximum possible body surface area for effective phototherapy; Brilliance Pro's specifications sheet showcases a uniform irradiance of ?45µW/cm2/nm.At its debut in December 2014, Brilliance Pro won the Best in Show award at NEOCON2014, India's largest neonatal conference hosted by the country's National Neonatology Forum, winning across all product categories and prices.
Safety
Unknown
Complementary Technical Systems
An optional Brilliance Pro Light Meter accessory exists for the Brilliance Pro that will allow doctors to measure the dose of light being delivered to an infant in real-time. D-Rev's Light Meter, usable with any Brilliance Pro unit, has been promoted to retail for less than 1/10th of the price of current light meters (cited as $1,500 USD), though the exact cost is to be determined.
Academic Research and References
Cline B.K., Vreman, H.J., Lou, H., Donaldson, K.M., Bhutani, V.K. Evaluation of an Affordable LED Phototherapy Device for Resource-Limited Settings. Poster presented at: 2011 Pediatric Academic Societies Conference.Cline, B.K., et al., Phototherapy Device Effectiveness in Nigeria: Irradiance Assessment and Potential for Improvement. Journal of Tropical Pediatrics, 2013. 59(4): p. 321-325
Phoenix Medical Systems, “Infant therapy – Brilliance Pro“, 2022.
Poplin, Kyle. “With Sales Up, D-Rev Doubles Down (Part 1): Launch of Upgraded Phototherapy Unit Brings Hopes of Expanded Market.” NextBillion, 2020.
VanHemert, Kyle. “How a Design Firm Is Saving Lives by Sweating the Details.” Wired, 2015.
D-REV and Phoenix Inc. “Brilliance pro Technical Details“, 2016
Compliance with regulations
Brilliance Pro's CE mark is pending, which will certify that the product meets European Union standards for safety and quality.
The manufacturer and distributor Phoenix Medical Systems also has ISO certification and pursues other standards for its products.
Evaluation methods
Unknown
Other Information
None.

Agriculture
February 21, 2024
Implemented by
Equalize Health

Agriculture
January 19, 2024
Implemented by
Greenlight Planet

Agriculture
January 11, 2024
Implemented by
Precious Plastic

Agriculture
June 24, 2024
Implemented by
RugGear

Agriculture
January 18, 2024
Implemented by
Sun King

Agriculture
February 20, 2024
Implemented by
SurgiBox

Agriculture
June 29, 2024
Agriculture
February 27, 2024
Implemented by
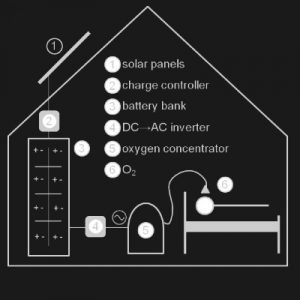
Solar Oxygen

Agriculture
May 20, 2024

Agriculture
February 26, 2024
Implemented by
Gabriela Galilea
Have thoughts on how we can improve?
Give Us Feedback
Mitra Ardron says:
Does anyone know the price of the silver impregnate bulb. The cost of the device itself is reasonable, but its quite likely that the real cost is the bulb itself, and there is no number on that?