Agriculture
November 24, 2023
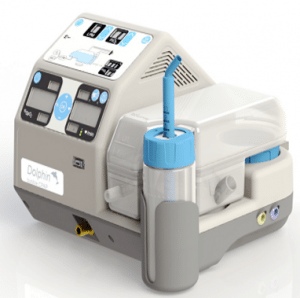
MTTS Dolphin CPAP
Read SolutionImplemented by
Dolphin CPAP
Updated on September 27, 2024
·Created on May 25, 2016
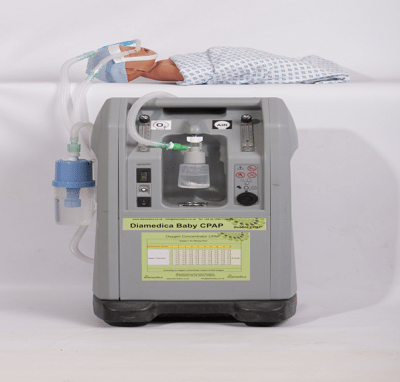
A gas-less CPAP machine for neonates.
The Diamedica Baby CPAP is an oxygen concentrator driven continuous positive airway pressure system (CPAP) that does not require compressed gases. This product was selected for inclusion in WHO’s 2013 Compendium of Innovative Health Technologies for Low-Resource Settings.
Target Regions
Africa
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$3,900.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises
Distributors / Implementing Organizations
Most Baby CPAPs are sold and distributed by Diamedica. Diamedica also has a partner distributor in East Africa, Joh Achelis & Söhne GmbH, that sells the Baby CPAP in Kenya, Tanzania, Uganda, Rwanda, Burundi, and Zambia.
Regions
Africa
Manufacturing/Building Method
The product is designed and manufactured in the UK.
Intellectural Property Type
Select Type
User Provision Model
NGOs typically purchase the Baby CPAP directly from Diamedica. The product is also available for purchase from a partner distributor in East Africa. The distributor typically sells the product to centralized procurement groups within government, private health systems, or church hospitals that subsequently distribute the product to a number of hospitals.Interview with distributor
Distributions to Date Status
Joh Achelis & Söhne GmbH, the East African distributor, has sold approximately 20-50 Diamedica products in the category of CPAP and oxygen concentrators up until 2016.Interview with distributor The quantity of Baby CPAPs distributed by Diamedica is unknown.
Design Specifications
This device generates and delivers a safe, easily controllable mixture of humidified oxygen and air for CPAP treatment. It is driven by an oxygen concentrator that has been modified to produce an increased output with a variable concentration of oxygen. The concentrator has also been modified so that warm waste air from the concentrator's compressor is directed towards the humidifier bottle. The device delivers flows of up to 8L/min of both oxygen and air. An oxygen/air mixing chart is provided with the product which eliminates the need for an oxygen analyzer. The oxygen/air mixture passes over a water humidifier and is delivered to the patient via lightweight respiratory tubing to silicon nasal prongs or a face mask. Pressure in the system is controlled by a bubble bottle that depends on the depth of tubing under water which is set by a simple dial on the bottle top.
The Baby CPAP requires time to calibrate before use. A yellow light will turn on after turning on the concentrator. This light will not turn off until the concentration of the gas supply reaches or exceeds 85%, indicating that the concentrator is working properly and ready for use.
The CPAP circuit (inspiratory and expiratory limb, bottle and lid and connections) should be cleaned after every patient or weekly if the same child is using the device for over a week. The circuit should be cleaned with soap before it is disinfected with sodium hypochlorite 0.05% or household bleach that is diluted to 0.05% hypochlorite. The exterior of the oxygen concentrator should be cleaned with a mild disinfecting cleaning agent or a diluted solution of bleach (usually 5.25% sodium hypochlorite). Instructions on how to properly clean the device are provided in the user manual. The Baby CPAP requires approximately 30 minutes of routine cleaning and maintenance each week.
Dimensions: 39 x 44 x 84 cm;
Weight: 26 kg
Technical Support
Diamedica offers free technical support via email, Skype, or telephone for the lifetime of the product. Diamedica also provides training for medical and technical personnel. Joh Achelis & Söhne GmbH, the East African distributor of the product, has engineers and technicians that provide full in-country technical support.Interview with distributor
Replacement Components
Almost all components in the device can be replaced.
Lifecycle
Unknown.
Manufacturer Specified Performance Parameters
This device is unique in being driven by its own oxygen concentrator which generates oxygen from atmospheric air and thus eliminates the need for expensive cylinders of compressed gases. The Baby CPAP can supply high flows of both compressed air and oxygen at a cost of .11 USD per hour, a fraction of the cost necessary when using oxygen cylinders.Interview with distributor
Vetted Performance Status
A clinical study conducted in Lilongwe, Malawi with 116 children with severe pneumonia reported a survival rate of 64% when the Baby CPAP was used for treatment.
Safety
When setting up the Baby CPAP, make sure that the device is positioned at least 30 cm away from walls and curtains so that the inlet opening is not obstructed. Children that are being treated with CPAP and are clinically stable (have no emergency signs and SpO2 >90%) should be disconnected from CPAP for 10–15 minutes each day in order for nursing staff to determine whether supplemental oxygen and CPAP is still required. The child should be immediately restarted on oxygen if severe hypoxaemia (SpO2 <80%), apnoea or severe respiratory distress occurs while the child is disconnected. Children with acute respiratory disease should not be discharged
until their SpO2 has been stable at 90% or more while breathing room air for at least 24 hours,
until danger signs have resolved, and until appropriate home treatment can be organized.Interview with distributor
Complementary Technical Systems
A pulse oximeter and a reliable power source are required.
Academic Research and References
G. Justus Hofmeyr et al., Obstetric care in low-resource settings: What, who, and how to overcome challenges to scale up?, in International Journal of Gynecology and Obstetrics, 2009, vol. 107, no. SUPPL., pp. S21–S45, doi: 10.1016/j.ijgo.2009.07.017.
J. E. Lawn, K. Kerber, C. Enweronu-Laryea, and O. M. Bateman, Newborn survival in low resource settings – Are we delivering?, BJOG: An International Journal of Obstetrics and Gynaecology, vol. 116, no. SUPPL. 1. John Wiley & Sons, Ltd, pp. 49–59, Oct-2009, doi: 10.1111/j.1471-0528.2009.02328.x.
C. E. Cox, S. S. Carson, J. H. Lindquist, M. K. Olsen, J. A. Govert, and L. Chelluri, Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: A prospective cohort study, Crit. Care, vol. 11, no. 1, pp. 1–11, Jan. 2007, doi: 10.1186/cc5667.
M. Unroe et al., One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A cohort study, Ann. Intern. Med., vol. 153, no. 3, pp. 167–175, Aug. 2010, doi: 10.7326/0003-4819-153-3-201008030-00007.
M. A. Rojas et al., Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: A randomized, controlled trial, Pediatrics, vol. 123, no. 1, pp. 137–142, Jan. 2009, doi: 10.1542/peds.2007-3501.
Compliance with regulations
CE certified
Evaluation methods
Clinical trials
Other Information
Selected as the runner up for the AAGBI Prize for Innovation in Anaesthesia, Critical Care, and Pain in 2014. Exhibitor at 2016 Geneva Health Forum.

Agriculture
November 24, 2023
Implemented by
Dolphin CPAP

Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
September 27, 2024
Implemented by
MamaOpe

Agriculture
December 4, 2023
Implemented by
Cure Tunisia

Agriculture
June 7, 2024
Implemented by
Bahmni Coalition

Agriculture
January 8, 2024
Implemented by
YakuPura

Agriculture
September 27, 2024
Implemented by
Biospectal

Agriculture
January 2, 2024
Implemented by
Uzima

Agriculture
February 21, 2024
Implemented by
Flexmo

Agriculture
June 6, 2024
Implemented by
Hello Doctor
Have thoughts on how we can improve?
Give Us Feedback
Robert Hauck says:
The users of this product are specified as “public Sectorâ€. It does not specify if training of the public sector is required. Given that this product will require the loading of ampules into their appropriate containers a minimum of clear user instructions are required. Training processes and support materials need to be made available for the public. Ongoing application and service support will be required
How did this product make it in here? What’s the qualification or standard to make it onto E4C the manufacturer has promoted its use in development projects?
Kelley Elahi says:
Could change “does not require compressed gases” to “does not require a separate compressed gas source.”
Kelley Elahi says:
“The Baby CPAP requires time to calibrate before use.” Are you sure it is calibrating? Or is it just warming up? Typically oxygen concentrators need time to warm up.
Iana Aranda says:
As per Bob: NOx
technology requires a source of appropriate chemicals (4 consumables – ampule
(generates NO2), scrubber (absorbs NO2 gas), up to 8 acids, and up to 8
chemical indicators) are required. Total cost is $20 per set of disposables. Although in developed countries this is not a
significant issue in developing countries and in remote areas the availability
of these chemicals is limited. Other
components such as housings, covers, electronics and controls are available if
an appropriate supply chain is developed and maintained to provide these
materials at the appropriate cost, quality and delivery to support
production. Piping, housings and
containment of chemicals is required to support NOx sterilization.