Agriculture
March 11, 2024
SOMATOINFRA Diagnostic System
Read SolutionImplemented by
OrthoTrauma
Updated on February 14, 2024
·Created on August 27, 2015
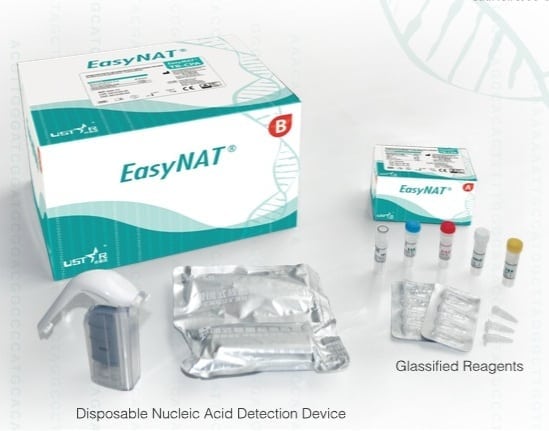
EasyNat TB-CPA Diagnostic Kit is an early screening tool for pulmonary tuberculosis (TB) for low resource countries.
The EasyNat TB-CPA Diagnostic Kit is designed as a screening tool for active pulmonary tuberculosis (TB) to improve the detection rate in people with suspected TB infection. The kit allows for rapid diagnostics that can be conducted without highly specific and expensive equipment as well as highly skilled staff. It is useful in many low-income settings where tuberculosis control is lacking.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$4.50
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Ustar Biotechnologies (Hangzhou) Ltd.
Countries
Canada, China, India, Indonesia, Philippines, Singapore, South Africa, United States
Manufacturing/Building Method
The product is mass-produced by Ustar Biotechnologies (Hangzhou) Ltd. in China.
Intellectural Property Type
Patent
User Provision Model
Product can be purchased from distributors in their respective countries Interview with representative
Distributions to Date Status
15,000 test strips as of July 2015 Interview with representative
Consumables
Disposable micropipette tips, 6.5-10ml centrifuge tubes, 1.5ml centrifuge tubes, with safe-lock feature, saline
Detection sensitivity
87%
Indispensable equipment for function (Y/N)
Micropipette, centrifuge, vortex, heating block or water bath, timer
Maintenance or calibration required by user at time of use? (Y/N)
Unknown
Number of Tests Performed
20 per box
Time required for procedure (minutes)
120
Design Specifications
The EasyNAT TB kit relies on Ustar's microbe detection platform which integrates CPA amplification technology and a disposable, contamination-proof DNA detection device. The testing involves three steps: 1. Liquifiy sputum and extract DNA to produce an amplification template and add it into in a 0.2 ml Eppendorff tube containing the CPA reagents; 2. Put the tube into a heat block maintaining 63? for one-hour for isothermal amplification 3. Insert the amplified tube into the Ustar disposable DNA detection device for qualitative read-out in 15 minutes. The turn-around time is about 3 hours from sample retrieval to results and provides a clear, 'positive/negative' read-out on the paper test strips.
Kit components include: 1. Disposable Nucleic Acid Extraction Device (with 3ml Syringe) x 20 pieces 2. 1ml Syringe x 20 pieces 3. Lysis Buffer - 15ml x 1 bottle 4. Wash Buffer 50ml x 1 bottle 5. Elution Buffer 3ml x 1 bottlle 6. 5ml Vial x 20 vials 7. 2ml Tube x 20 tubes 8. 0.6ml Tube x 20 tubes
Kit Weight: 500g/20 tests. There are two boxes for one TB kit, Kit A: 9.5cm x 6.5cm x 5.5cm, 53g; and Kit B:25cm x 19cm x 15cm, 1.2kg. Interview with representative
Technical Support
Provided by the manufacturer
Replacement Components
Ustar Biotechnologies provides reagents in liquid form or glassified form on request.
Lifecycle
Single-use, disposable tests Interview with representative
Manufacturer Specified Performance Parameters
Testing turn-around time is about 3 hours. The amplification and DNA detection process takes place in a sealed-off condition thus preventing cross-contamination. EasyNAT TB detects as low level as 10 pathogens per 1 ml. It is fast, easy-to-use, and requires no costly qPCR machine and PCR infrastructure, no cold-chain required. Interview with representative
Vetted Performance Status
In a large clinical trial ran by the Chinese Anti-tuberculosis Association at 4 county-level TB hospitals in China (n=2,200), EasyNAT TB proved: 1) high sensitivity 84.1% & Specificity 97.8%, using L-J solid media and 16S rRNA sequencing results as the gold standard; 2) Sensitivity 59.8% in smear negative samples; 3) recommended as a rapid screening test for active TB as it reduced turn-around time from 54 days to 4-5 hours. Interview with representative The Ifakara Health Institute conducted a 147-participant study in Tanzania and concluded that the EasyNAT assay has a 66.7% sensitivity.
Safety
No listed hazards but the kit uses frozen materials and temperature controlled transport is important for effective use.
Complementary Technical Systems
Additional equipment is required in protocol and not provided with product. This includes: isothermal metal heater or water bath, vortex mixer, micropipette and disposable tips, timer, and a centrifuge (optional).
Academic Research and References
Fang R, et al., Cross- priming amplification for rapid detection of Mycobacterium tuberculosis in sputum specimens. J Clin Microbiol 2009;47:845-847.
Michael S. Cordray and Rebecca R. Richards-Kortum. Review: Emerging Nucleic Acid–Based Tests for Point-of-Care Detection of Malaria. The American Society of Tropical Medicine and Hygiene; 87(2): 223-230. doi:10.4269/ajtmh.2012.11-0685
Xichao Ou et al., A multicenter study of Cross-Priming Amplification for tuberculosis diagnosis at peripheral level in China. Elsevier: Tuberculosis. 2014
Mhimbira FA, Detection of Mycobacterium tuberculosis by EasyNAT diagnostic kit in sputum samples from Tanzania. J Clin Microbiol. 2015 Apr;53(4):1342-4. doi: 10.1128/JCM.03037-14. Epub 2015 Jan 21.
McNerneya Ruth, New tuberculosis diagnostics and rollout International Journal of Infectious Diseases, Volume 32, March 2015, Pages 81–86
S. Deng et al., Accuracy of Commercial Molecular Diagnostics for the Detection of Pulmonary Tuberculosis in China: A Systematic Review., Sci. Rep., vol. 9, no. 1, pp. 1–11, Dec. 2019, doi: 10.1038/s41598-019-41074-8.
IF Design Award, “EasyNAT TB CPA Kit“, 2014
Quimin You, Hangzhou, “ENCLOSED UNIT FOR RAPID DETECTION OF A TARGET NUCLECACID AMPLIFICATION PRODUCT“, US 8,415,141 B2, United States Patent, 2013
Compliance with regulations
EasyNAT TB had also undergone verification testing at China National Institute for Controls of Pharmaceutical and Biological Products (NICPBP) in Beijing in 2008. Regulatory approval details unknown.
Evaluation methods
Clinical evaluation conducted at Shanghai Centers for Disease Control from March to June of 2008. EasyNAT TB had also undergone verification testing at China National Institute for Controls of Pharmaceutical and Biological Products (NICPBP) in Beijing in 2008. EasyNAT TB passed national reference panel with lowest detection level of 10 bac. copy/ml.
Other Information
None

Agriculture
March 11, 2024
Implemented by
OrthoTrauma
Agriculture
February 26, 2024
Implemented by
Wyss Institute

Agriculture
February 26, 2024
Implemented by
Hemex Health

Agriculture
December 14, 2023
Implemented by
AFRIpads

Agriculture
January 17, 2024
Implemented by
Aquagenx
Agriculture
November 17, 2023
Implemented by
AYZH

Agriculture
January 18, 2024
Implemented by
Barry Lloyd, University of Surrey

Agriculture
September 27, 2024
Implemented by
PATH

Agriculture
December 30, 2023
Implemented by
B Medical Systems

Agriculture
June 17, 2024
Implemented by
New America
Have thoughts on how we can improve?
Give Us Feedback
Manufacturing/building method: According to photos on their Facebook page, the bio-bags are manufactured in Belgium (https://www.facebook.com/ba… ) and the frames are manufactured in Taiwan (https://www.facebook.com/ba… ).
An isothermal NAT to determine TB is a useful technology. The ability to monitor TB accurately and safely will help physicians and health care providers detect infection and manage therapy. The TB device as implemented by Ustar Biotechnologies is promising but with shortcomings.
The requirement for the user to provide a heat source to heat water to process samples and for nucleic acid amplification limits portability to remote locations. The requirement for cold chain for critical enzymes is also a limitation to usage in remote locations.
Additionally, since multiple steps need to be performed to run the kit, operators will need appropriate training. As the device will be tethered to a facility with the necessary systems to operate the test, this may be a moderate limitation to deployment.