
Agriculture
February 5, 2024
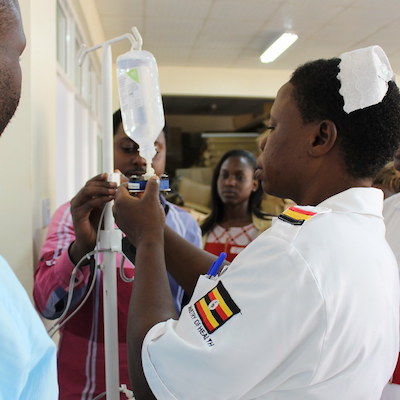
DripAssist Infusion Rate Monitor
Read SolutionImplemented by
Shift Labs
Updated on December 4, 2023
·Created on October 3, 2020
An infusion controller that monitors and regulates intravenous fluids or medication during therapy in patients.
The Electronically Controlled Gravity Feed Infusion set features dynamic flow control that monitors and regulates intravenous fluids or medication during therapy. It is an add-on device that is applicable with existing intravenous drip sets.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$100.00
Target Users (Target Impact Group)
Household
Distributors / Implementing Organizations
Uganda Industrial Research Institute
Countries
Rwanda, Uganda
Manufacturing/Building Method
The ECGF device prototypes were manufactured in Uganda. The device is still in prototype phase and not yet manufactured at scale.
Intellectural Property Type
Select Type
User Provision Model
The device is still in prototyping phase and so the designers have not yet selected their user provision model.
Distributions to Date Status
To date, 5 prototypes have been manufactured and used for clinical testing. There are no market distribution for the product yet.
Delivery depths
Intravenous delivery
Maximum injection volume (mL)
N/A
Design Specifications
The ECGF device is designed to accurately administer fluids and other therapeutics intravenously by controlling the rate of fluid flow based on feedback from a drop sensor. An actuator is used to control the flow of fluid. The device uses both a mains and solar battery. The device automates the regulation of fluid and drug delivery and removes the need for manual regulation, as is the current standard in Uganda. To set the desired flow rate, the user uses the device keypad to type in the desired flow rate.
Technical Support
Provided by the manufacturer.
Replacement Components
The device battery must be replaced after is maximum recharge cycles.
Lifecycle
Battery life is approximately 8 hours.
Manufacturer Specified Performance Parameters
Manufacturer specified performance targets include safety, accuracy, ease of use, and maintainability.
Vetted Performance Status
5 prototype devices were built and pre-clinically tested in 2017. After the initial testing, clinicians were trained to install and operate the ECGF device where the time required for clinicians to regulate and monitor fluids was studied. During the clinical trial, 79 nurses, doctors, and biomedical technicians from 3 health facilities were trained on the ECGF device. Both user experience data, such as ease of set up, usability, functionality, safety, and ergonomics, as well as analytical data was collected. The analytical data focused on flow rate accuracy. In the first human pilot consisting of 12 adults, the device achieved a flow rate accuracy (ie. percentage error margin between the prescribed and actual dosages) of +/- 7%. A clinical safety trial for children aged 5-8 was conducted and found that the most notable improvements by the ECGF device were in children suffering from malaria, dehydration, severe diarrhea, and pneumonia. A final clinical trial for children aged up to 4 years was completed in early February 2019 and the analysis is being finalized. The clinical trials had 160 participating children.
Safety
The device features several safety systems such as alarms for rate of infusion (rapid or slow), total volume, and faulty sensors.
A safety trial was conducted with adults and children. The team is improving the ECGF design to further improve the flow rate regulation.
Complementary Technical Systems
The ECGF device is compatible with existing intravenous drips.
Academic Research and References
P. N., Makobore and M., Mulerwa, 2019, An Electronically Controlled Gravity Feed Infusion Set for Intravenous Fluids, Biomed. Eng. Africa, pp. 124–138.
Compliance with regulations
The designers are planning to obtain CE marking for the device.
Evaluation methods
The product has been evaluated for flow rate accuracy, ergonomics, safety, and ease of use.
Other Information
The development of the ECGF device was sponsored by Grand Challenges Canada in the Stars in Global Health program from 2017-2018. The ECGF device won second prize in the Health and Wellbeing Innovations Category at the 2017 IPA Awards in Accra, Ghana.

Agriculture
February 5, 2024
Implemented by
Shift Labs

Agriculture
December 7, 2023
Implemented by
Envirogard Products Ltd

Agriculture
January 30, 2024
Implemented by
Danny Wright

Agriculture
December 27, 2023
Implemented by
Aquabox, UK

Agriculture
December 18, 2023
Implemented by
Be Girl

Agriculture
January 11, 2024
Implemented by
Kenya Ceramic Project (KCP)

Agriculture
January 8, 2024
Implemented by
DayOne Response

Agriculture
February 26, 2024
Implemented by
Hemex Health
Agriculture
February 27, 2024
Implemented by
Mbarara University of Science and Technology

Agriculture
June 26, 2024
Implemented by
Helmholtz Centre for Infection Research (HZI)
Have thoughts on how we can improve?
Give Us Feedback