
Agriculture
December 19, 2023
Ecodry Urine-Diverting Dry Toilet
Read SolutionImplemented by
Wostman
Updated on February 15, 2024
·Created on August 6, 2016
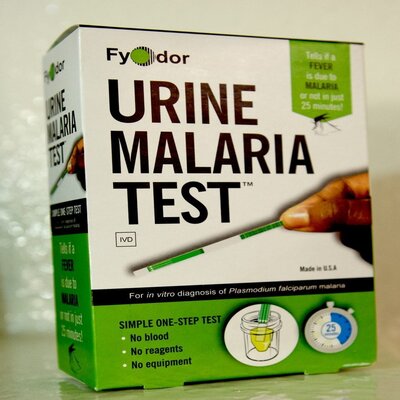
Simple Urine based malaria test dipstick
Fyodor’s Urine Malaria Test (UMT) is a novel commercial dipstick technology for rapid point-of-need diagnosis of clinical malaria from urine instead of blood.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$7.00
Target Users (Target Impact Group)
Household, Community, Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
m-medix (Online Pharmacy and drug store)
Competitive Landscape
Direct competitors include DDG Rapid Assessment of Infectious Diseases (RAID) Device.
Regions
South America, Southeast Asia, Sub-Saharan Africa
Manufacturing/Building Method
Fyodor Urine Malaria Test kit is mass-produced in Baltimore, Maryland United States by the Fyodor Biotechnologies Inc and is supplied around the world mainly in the Sub-Saharan region and the Tropics.
Intellectural Property Type
Patent
User Provision Model
User can acquire the test strips from the developers, online pharmacies like m-medix and other local pharmacies mostly in Nigeria
Distributions to Date Status
Unknown
Consumables
Unknown
Detection sensitivity
125 parasites/µl
Indispensable equipment for function (Y/N)
No Equipment
Maintenance or calibration required by user at time of use? (Y/N)
Yes, Clean container
Number of Tests Performed
1
Power supply type: Continuous, Recharging only (V, time required, battery life), Other
None
Time required for procedure (minutes)
20
Design Specifications
Most generally, the Fyodor Urine Malaria strip is based on Lateral flow techniques. At the Immunological level, clinical malaria commonly results in elevated levels of Histidine Rich Protein-2 and or its protein fragments in the patient’s urine. Against this background, cognate recombinant monoclonal antibody reagents were developed by Fyodor. This qualitative assay consists of a nitrocellulose membrane strip containing relevant antibody reagents and controls immobilized at the specific individual site on the membrane. Dipping the immunochromatographic dipstick in urine, specific malaria parasite protein present in the urine migrates and interacts with immobilized cognate monoclonal antibody resulting in dark-colored strips on the dipstick. UMT strips are packaged in a sealed Mylar foil pouch with a desiccant and stored at room temperature.
The UMT is a sensitive and specific immunochromatographic lateral flow assay that can be easily performed by an untrained individual using a urine sample. To perform, the test strip is dropped into a clean container with as little as 100 µl (5 drops) of urine, allowed to wick up the strip for 1-2 minutes, and incubated at room temperature for 20 minutes.
Results
Positive (+) for malaria is indicated by two red lines on the test strips; Negative (-) for malaria is shown by one red line on the test strip; Invalid result is shown when there is no line on the test strip, in this case, you have to repeat the test.
Sensitivity: Adults: 85% | Children ?5: 93%, Specificity: Adults: 84% | Children ?5: 83%.
Technical Support
In the packaging, the developer includes the guide notice for users.
Third-party organizations like m-medx also published these user guides.
Replacement Components
No components are needed since each pack comes with a dipstick fully packed and sealed for single use.
Lifecycle
This product is for one-time use.
Manufacturer Specified Performance Parameters
According to Fyodor, the UMT demonstrates equivalent performance compared to commercially available blood-based rapid tests for the diagnosis of clinical malaria, with a limit of detection of 125 parasites/µl, well within the 100-200 parasites/µl analytical performance range recommended for malaria RDT’s by the World Health Organization.
Among the performance targets set were:
Vetted Performance Status
A 2014 study in which the disease prevalence was 41.2% found that the detection sensitivity for the UMT was 83.75%. In addition, the UMT had a sensitivity of 120 parasites/uL but the sensitivity at parasite density less than 200 parasites/uL was 50% and 89.71% at density ?201 parasites/uL with a specificity of 83.48%. The positive and negative predictive values were 77.91% and 88.07%, respectively. Ultimately, the study concluded that the UMT showed a moderate level of sensitivity compared with blood smear microscopy. The test kit requires further improvement on its sensitivity to be deployable for field use in malaria-endemic regions. An additional clinical trial was conducted in 2014.
Safety
The Fyodor Urine Malaria strip is a class device under the category of measuring devices. Although not obliged to meet stringent conformity protocols the developers ensure that they comply with the requirements through clinical trials and their protocol. This means that, although the device does not get into contact and affect the human body part, it can provide dependable results for a prescription.
Complementary Technical Systems
In the packaging, there is a user manual with guidelines on how to use the dipstick
Academic Research and References
Oyibo, W. A., Ezeigwe, N., Ntadom, G., Oladosu, O. O., Rainwater-Loveth, K., O’Meara, W., … & Brieger, W., 2017, “Multicenter pivotal clinical trial of urine malaria test for rapid diagnosis of Plasmodium falciparum malaria,” Journal of clinical microbiology, 55(1), pp. 253-263.
Oguonu, T., Shu, E., Ezeonwu, B. U., Lige, B., Derrick, A., Umeh, R. E., & Agbo, E., 2014, “The performance evaluation of a urine malaria test (UMT) kit for the diagnosis of malaria in individuals with fever in south-east Nigeria: cross-sectional analytical study,” Malaria Journal, 13(1), pp. 1-6.
Okete, J. A., Oden, E. M., & Adofikwu, C. E., 2018, “Reliability of Urine Malaria Test (UMT) for Malaria Diagnosis,” Asian Journal of Research in Medical and Pharmaceutical Sciences, pp.1-9.
Nuru, J., 2017, “MALARIA DIAGNOSIS: CAN THE FUTURE BEAT THE STATUS QUO?.”
Godwin, A. A., Sulieman, M. M., & Panda, S. M., 2017, “Efficiency of Urine Malaria Test Kit for the Diagnosis of Malaria of Febrile Patients in Gombe, Nigeria.”
M-medix, “Fyodor Urine Malaria Test x5 Strips”
Ojomo, Efosa., “Disrupting malaria: How Fyodor Biotechnologies is changing the game“, World Bank Blogs.
Fyodor Biotechnologies, “Clinical Performance Evaluation of Fyodor Urine Malaria Test (UMT)“, National Center for Biotechnology Information, 2014
Compliance with regulations
Yes. The manufacturer ensured that the product is up to standard and tested to meet the WHO requirements.
Evaluation methods
An observational cross-sectional diagnostic test study as well as case-control, prospective clinical trial was conducted. Detailed methods can be found on each link.
Summary of the results:
- Sensitivity for the UMT was 83.75%
- Specificity was 83.48%
- The positive predictive value was 77.91%
- The negative predictive value was 88.07%
Another multicenter evaluation was carried out by Wellington A. Oyibo
Other Information
In news, the World Bank article published as a blog in 2016 acknowledges Fyodor Biotechnologies as a company disrupting Malaria testing.
Another article by the Swiss Re Foundation publishes the UMT as the first non-blood malaria test technique.

Agriculture
December 19, 2023
Implemented by
Wostman

Agriculture
January 17, 2024
Implemented by
Aquagenx

Agriculture
February 14, 2024
Implemented by
Diagnostics for all

Agriculture
January 17, 2024
Implemented by
mWater

Agriculture
January 24, 2024
Implemented by
Nanyang Technological University (NTU) Singapore

Agriculture
January 17, 2024
Implemented by
HACH

Agriculture
September 7, 2025
Implemented by
Karolinska Institutet
Agriculture
February 26, 2024
Implemented by
Wyss Institute

Agriculture
January 3, 2024
Implemented by
Cascade Designers, Inc

Agriculture
January 17, 2024
Implemented by
Kärcher Futuretech
Have thoughts on how we can improve?
Give Us Feedback
Not much to add to this product.
A general concern for urine (or oral fluid) diagnostics is that the state of hydration contributes to the quality of the sample. Urine is tricky…to much fluid will dilute the sample. Dehydration will concentrate the sample. There needs to be a normalization to a well known marker such as creatinine.
A malaria RDT focused on urine is a welcome addition to the malaria detection armamentarium. There is wide appeal to move away from collection of blood as a sample matrix (local superstitions need to be overcome). The use of urine as a test matrix may help overcome patient reluctance.
In the field, the device was shown to detect malaria antigen at “moderate level of sensitivity compared with blood smear microscopy”. The is a compelling start and will likely improve with more assay development.
The primary issue with urine (or oral fluid) as a test matrix is the variability of the sample. The state of hydration will influence matrix concentration. This is potentially problematic. In severe cases of dehydration, sample acquisition may be impossible, especially with children. The problem will be exacerbated in warmer climates. If the developers can implement a way to control for sample heterogeneity, and sensitivity, the device will be easily embraced.
The ‘distribution to date’ field could benefit from a ‘user picture upload’ portal eventually?