
Agriculture
November 22, 2024
AEST Agricultural Waste Charcoal Briquettes
Read SolutionImplemented by
Appropriate Energy Saving Technologies Limited (AEST)

Updated on February 8, 2024
·Created on June 30, 2016
The LOGIQ V2 is a high-performance, compact, laptop-based ultrasound imaging system.
The LOGIQ V2 is a high-performance, compact, laptop-based ultrasound imaging system. This system provides ultrasound imaging in all aspects and is not limited to general imaging and obstetrics applications. Information for this product was provided courtesy of the WHO’s 2016 Call for Innovative Health Technologies for Low-Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$4,700.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
GE Healthcare, National Ultrasound, Innovative Medical Technologies
Competitive Landscape
Direct competitors include Clarius Ultrasound, Healcerion Sonon 300C, dDopp, and GE VScan Pocket Ultrasound.
Regions
Worldwide
Manufacturing/Building Method
The LOGIQ V2 is currently manufactured in China.
Intellectural Property Type
Patent
User Provision Model
Customers can fill out the contact form on the GE Healthcare website to request a quote. The product is also sold through "National Ultrasound" and "Innovative Medical Technologies". The Logiq V2 can also be purchased at regional and local manufacturer offices.
Distributions to Date Status
Between 101-1,000 units have been distributed. The exact amount of units distributed is unknown.
Power supply type
100-120 V / 220-240 VAC, rechargeable lithium ion battery (4100 mAh)
Consumables
Ultrasound gel.
Indispensable equipment for function (Y/N)
Probe(s).
Maintenance or calibration required by user at time of use? (Y/N)
No.
Scanning modes
2D, M, Color Flow, Pulsed Wave, Power Doppler
Clinical application
Fetal/OB, Abdominal, Pediatric, Urology, Cardiac (adult & pediatric), Peripheral Vessel, Small Organ, Vascular, Transcranial, Peripheral Vessel, Neonatal and Adult Cephalic, Musculoskeletal Conventional & Superficial, Transrectal, Transvaginal, interventional procedures (e.g. Nerve Block, Vascular Access, Tissue Biopsy/Fluid Drainage)
Display
Unknown.
Probe frequency (MHz)
Varies.
Design Specifications
The LOGIQ V2 produces high quality images using CrossXBeam (Spatial Compounding), SRI HD (Speckle Reduction Imaging in High Definition), and Coded Harmonics. The system includes workflow features such as Raw Data for post-processing of images, Automatic Optimization, Virtual Convex, and B-Steer, which maneuvers the ultrasound beam for better visualization of the needle (available on linear probes). The system includes SonoBiometry software that allows for automatic measurement of key fetal biometry measurements. The system also includes ScanCoach, a reference tool that can be used during living imaging that displays reference images, animations, and schematics to guide the proper probe placement.
The system can function via wall electrical supply or with a rechargeable battery. The battery life is 45 minutes and the charging time is 2.5 hours. The LOGIQ V2 system is compatible with 7 ultrasound probes that each have different frequency bandwidths and clinical applications.
Proprietary software is included with the system and is available in 14 different languages. Key functionality of the software include operation system, database server, conversion reports into PDF format, data export and import with CD/DVD.
Dimensions: 120x368x396mm;
Weight: 6kg
Temperature/humidity requirements :10-40°C/30-80% RH
Technical Support
Training is provided by a manufacturer representative. The extent of the training providing depends on the user's current knowledge of ultrasound. The system is also equipped with on-board training modules that provide information on settings, operation, connectivity, and maintenance. This feature can be used in areas without internet access.
The user can perform daily routine maintenance on the system with guidance from the on-board training modules. The system requires yearly on-site maintenance by manufacturer technicians or authorized service providers.
Purchase of the device includes a 3 year warranty that includes probes, parts, and labor.
Replacement Components
Spare parts can be purchased from GE or its authorized service providers if the system is no longer covered under warranty.Interview with representative
Lifecycle
The estimated lifetime of the device is 5-10 years.
Manufacturer Specified Performance Parameters
Unknown.
Vetted Performance Status
Unknown.
Safety
Unknown.
Complementary Technical Systems
Unknown.
Academic Research and References
None.
Food and Drug Administration, “Indications of Use“, DEPARTMENT OF HEALTH AND HUMAN SERVICES, 2017
Compliance with regulations
AAMI/ANSI ES60601-1, IEC60601-1-2, IEC60601-2-37, NEMA UD 3, ISO10993-1, NEMA UD 2, ISO14971, NEMA Digital Imaging and Communications in Medicine (DICOM) Set (Radiology), MDS2, CE Marked, FDA 510(k), TUV certified, Japan MHLW, IHE compliant
Evaluation methods
The system has undergone usability testing with clinicians to evaluate ease of use. Other quality assurance measurements that were applied include risk analysis, requirements reviews, design reviews, testing on unit level (module verification), integration testing (system verification), performance testing, safety testing, and stimulated use testing. The technical capability of the equipment for intended objectives has been thoroughly evaluated both in the restricted laboratory environment and unrestricted field environment by GE Healthcare.
Clinical usability studies are being conducted in different institutional settings in Europe, USA, ASEAN, and Japan. (Interview with representative)
Other Information
Designers should also investigate additional guidelines, such as the WHO Manual of diagnostic ultrasound. Designers should also take social factors into consideration, such as the use of ultrasounds for gender-biased sex selection in some parts of the world.

Agriculture
November 22, 2024
Implemented by
Appropriate Energy Saving Technologies Limited (AEST)

Agriculture
February 5, 2024
Implemented by
Centre for Vision in the Developing World

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 1, 2024
Implemented by
Infantrust Parenting Solutions

Agriculture
February 16, 2024
Implemented by
Equalize Health
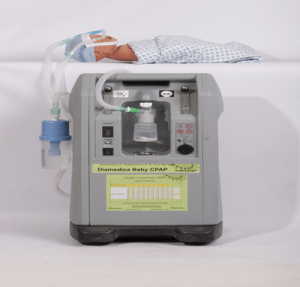
Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
December 3, 2024
Implemented by
Veryday (McKinsey Design)

Agriculture
February 29, 2024
Implemented by
Free Wheelchair Mission

Agriculture
December 3, 2024
Implemented by
Greenway
Have thoughts on how we can improve?
Give Us Feedback