
Agriculture
February 5, 2024
Gradian Health Systems Universal Anaesthesia Machine
Read SolutionImplemented by
Gradian Health Systems

Updated on February 2, 2024
·Created on June 19, 2020
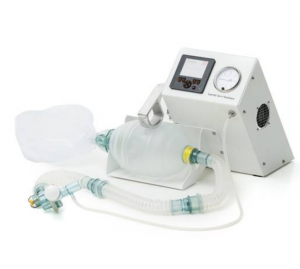
The Gradian CCV is a mechanical ventilation system meant for use in low-oxygen and electricity settings.
The Gradian Comprehensive Care Ventilator (CCV) is a mechanical ventilation system. It is meant to be used for critically ill patients in hospital settings and has a battery life of up to 21-hours. The machine is portable and features an internal air compressor.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
The product is distributed by the manufacturer. There are also distributors in several countries around the world: Homintec (Benin, Togo), Meditek SARL (Burkina Faso), HeiDoctor (Ghana), Labonet (Guinea), Avoma Group (Mozambique, Swaziland), Biomedical Engineering Foundation of Nepal (Nepal), Artemis Life (Nigeria), Medical Engineering Solutions, Ltd (Rwanda), Medical Equipment Management Systems (Sierra Leone), Kas Medics, Ltd (Tanzania), Joint Medical Store (Uganda), Sonergy Diagnostics, Ltd (Zambia).
Regions
Africa, Asia, Central America, North America, Oceania, South America
Manufacturing/Building Method
The product is manufactured in the United States and in the United Kingdom.
Installation of the product comes with training of clinical users and technicians at the facility where it is being installed.
Intellectural Property Type
Patent
User Provision Model
The product can be purchased directly from the manufacturer and from Medics Guide. Gradian has distributors in several countries in Africa, Central America, and Asia.
Distributions to Date Status
As of December 2019, over 200 units have been distributed. Due to the COVID-19 epidemic the distributions have likely increased.
Design Specifications
The Gradian CCV is a ventilator meant for use in a variety of situations. In short, the product consists of the central control system with various accessories for ease of use. The product has a 7-hour battery life and comes with a battery pack that can add up to 14 hours of battery life, giving a total of 21 hours of battery life. There is a hook on the central control system that allows the system to be hung, and the product also has a rolling stand for ease of transport. The user interface consists of several digital displays. There is an internal air compressor that can be used when external sources are not accessible. There is also an oxygen reservoir that can be used with an oxygen concentrator or flowmeter if a pipeline or cylinders are unavailable. The system allows the operator to pre-program custom ventilation mode settings for specific disease conditions.
The product dimensions are: 34 x 13 x 35 cm. The system weight is 8.5 kg. The product has an ABS casing and has ingress protection compliant with IP22 and has a reusable silicone breathing circuit.
Technical Support
Provided by the manufacturer. Each country with a product distributor has a team of biomedical engineers and technicians that are available to provide technical support.
Replacement Components
The particle filter should be checked every 3 months and replaced if visibly dirty. Batteries should be replaced every 3 years. HME (heat and moisture exchanger) filters should be discarded between patients.
Lifecycle
The lifetime of the product is 6+ years and requires comprehensive maintenance after 6 years to ensure proper function and safety. The product comes with a 3 year warranty which covers remote support, yearly preventative maintenance, and repairs of non-consumable parts due to manufacturing defects.
Manufacturer Specified Performance Parameters
Manufacturer specified performance targets include: multi-environment use, broad-featured capability, ease of use, low cost ownership, and reliable and local customer support.
Vetted Performance Status
The product has ingress protection to IP22, it is shock tested to 100 G-force, and is vibration-tested to IEC 60068-2-6.
Safety
The CCV User Guide offers substantial information regarding the safety of the product and should be consulted for a complete understanding of the necessary safety precautions.
Complementary Technical Systems
The product requires a reliable power source to for battery recharging. An alternate means of ventilation should be available while using the CCV in case of a mechanical or system problem.
Academic Research and References
A. O. Ademuyiwa et al., 2020 COVID-19 Preparedness within the Surgical, Obstetric and Anesthetic Ecosystem in Sub Saharan Africa, Ann. Surg., 272(1):e9-e13.
M. Shahid, 2019 Prototyping of artificial respiration machine using AMBU bag compression, in ICEIC 2019 – International Conference on Electronics, Information, and Communication, pp. 1-6.
Gradian Health Systems, “Video Explainer“, Vimeo.
Gradian Health Systems, “Global Presence“, Vimeo.
Compliance with regulations
The system is CE-certified and has been cleared by the U.S. FDA.
Evaluation methods
The product has ingress protection to IP22, it is shock tested to 100 G-force, and is vibration-tested to IEC 60068-2-6.
Other Information
Winner of the Saving Lives at Birth Award to improve maternal and newborn healthcare in Zambia.

Agriculture
February 5, 2024
Implemented by
Gradian Health Systems

Agriculture
February 15, 2024
Implemented by
Jana Care Inc.

Agriculture
September 27, 2024
Implemented by
Diamedica
Agriculture
March 8, 2024
Implemented by
Laerdal Global Health

Agriculture
March 8, 2024
Implemented by
MTTS

Agriculture
March 8, 2024
Implemented by
Mechanical Ventilator Milano

Agriculture
March 8, 2024
Implemented by
Conseil Européen pour la Recherche Nucléaire (CERN)
Agriculture
January 10, 2024
Implemented by
Ideas at Work

Agriculture
November 22, 2023
Implemented by
Laerdal Global Health

Agriculture
December 29, 2023
Implemented by
Lifesaver
Have thoughts on how we can improve?
Give Us Feedback