
Agriculture
February 2, 2024
Gradian Comprehensive Care Ventilator (CCV)
Read SolutionImplemented by
Gradian Health Systems
Updated on February 15, 2024
·Created on July 27, 2017
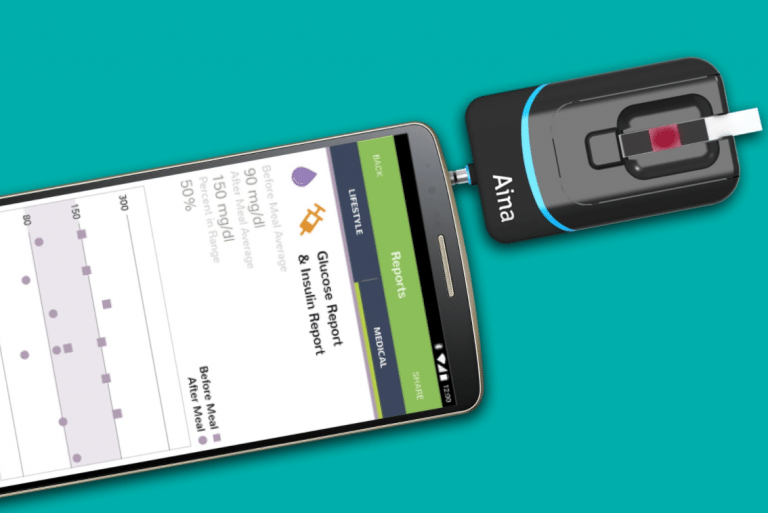
Aina is a diagnostic device on smartphones that can analyzes HbA1c, blood glucose, hemoglobin, creatinine, and lipids from a capillary blood sample.
Aina is a diagnostic sensor and smartphone application that plugs into any smartphone and analyzes HbA1c, blood glucose, hemoglobin, creatinine, and lipids from a capillary blood sample in only minutes. The device can be used by patients for self-monitoring as well as by healthcare providers for mass screening and point-of-care testing.
Aina has also developed the Habits Program, based on the landmark Diabetes Prevention Program, to deliver digital coaching and support to patients recently diagnosed with pre-diabetes or type II diabetes. The user can also visit Jana care’s desk support
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$50.00
Market Suggested Retail Price (Secondary Currency)
1.00
Target Users (Target Impact Group)
Household, Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
The manufacturer distributes his own product, Hospitals like Narayana Health Hospital in Bangalore, India are implementing the product
Competitive Landscape
Direct competitors include PATH: Strip test for gestational diabetes.
Regions
Europe
Countries
India, Malaysia, Singapore
Manufacturing/Building Method
The product is also Mass Produced. The company is headquartered in Boston with an ISO13485 certified manufacturing site in India
Intellectural Property Type
Patent
User Provision Model
Patients can procure the Jana Care Aina from health clinics and hospitals or purchase it directly from the manufacturer and download the companion app from the Google Play or the Apple Store. The healthcare workers also use the device to directly test patients.
Customers can also request a demo from the manufacturers website
Distributions to Date Status
50,000 patients in India as of February 2017.
Consumables
Testing strips
Detection sensitivity
Unknown
Indispensable equipment for function (Y/N)
Y
Maintenance or calibration required by user at time of use? (Y/N)
Y
Number of Tests Performed
6
Power supply type: Continuous, Recharging only (V, time required, battery life), Other
Smartphone battery
Battery: 1x 1.5V AAA Battery
Battery Life of at least 200 Tests
Time required for procedure (minutes)
Approximately 3 minutes
Design Specifications
The Jana Care Aina Device is a blood glucometer that uses existing colorimetric test strips and plugs into any smartphone via the headphone jack. Blood readings are communicated to an accompanying mobile app, which allows pre-diabetic users to monitor consistently their glucose levels as well as weight, food intake, and physical activity. The product works with iOs and Android devices.
The Aina device HbA1c test utilizes a method called boronate affinity. The Aina HbA1c Test Kit consists of test strips, reagents, wash buffers, capillary tubes for collection of samples, and pipette tips. The reagent contains a lysing agent and a blue boronic acid conjugate. When blood is added to the reagent, the erythrocytes are lysed and all hemoglobin precipitates. The boronic acid conjugates binds to the glycosylated hemoglobin. An aliquot of the reaction mixture is applied to the test strip and all the precipitated hemoglobin, conjugate-bound and unbound, remains on top of the filter. Any unbound boronate is removed with the wash buffer. The precipitate is evaluated by measuring the blue (glycosylated hemoglobin) and the red (total hemoglobin) color intensity
respectively with the Aina Device, the ratio between them being proportional to the percentage of the glycosylated hemoglobin in the sample.
The product has the following technical specifications
Dimensions: 79.57 (L) X 36.54 (B) X 16.40 mm (H)
Weight: 0.021kg (without battery), 0.032 kg (with battery)
Power Requirements: 1.5V AAA battery
Safety: Battery powered device, tested for compliance with EN 61010-1, EN 61010-2-101 and RoHS
EMC Emissions/Immunity: Aina Device complies with applicable EMC emission requirements as per EN 61326-1:2013 and EN 61326-2-6:2013
Technical Support
Users can be supported through the habits App. The user can also visit Jana care's desk support
Replacement Components
Single-use test strips
Lifecycle
The test strips are single-use. Aida device lifecycle is unknown.
Manufacturer Specified Performance Parameters
Goals:
- Early detection for diabetes, pre-diabetes and other chronic diseases
- In point diagnostics and monitoring
- Easy to use
- Low cost
Vetted Performance Status
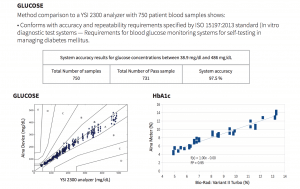
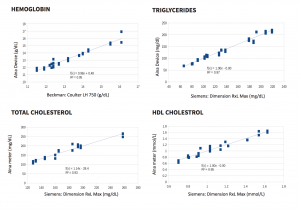
Method comparison to a YSI 2300 analyzer with 750 patient blood samples shows that the Jana Care Aina conforms with accuracy and repeatability requirements specified by ISO 15197:2013 standard (In vitro diagnostic test systems — Requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus). Additional test results can be found in the Analytical Performance Summary.
Safety
The device is tested for compliance with EN 61010-1, EN 61010-2-101 and RoHS
It is important to make sure hands and device are clean before use in order to prevent infection.
Complementary Technical Systems
Smartphone, testing strips, the Jana Care Habits app
Academic Research and References
Clarke, W. L., Cox, D., Gonder-Frederick, L. A., Carter, W., & Pohl, S. L. (1987). Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes care, 10(5), 622-628.
Bloom, Sonali., “Jana Care – Revolutionizing Diabetes Diagnosis and Treatment through Accessible Mobile Technology“, Digital Innitative, Harvard Bussiness School, 2015
RocketReach, “Jana Care Information”
Sidhant Jena and Michael Depa, “Portable medical diagnostic systems and methods using a mobile device“, US9241663B2, U.S. Patent, 2015
EPAM CONTINUUM, “JANA CARE: AINA DEVICE“, 2016
Jana Care Inc. “Indications of Use“, Food and Drug Administration, DEPARTMENT OF HEALTH AND HUMAN SERVICES, 2020
Cabrera, Marquis. “Harvard Grads Develop Tech to Help Patients Better Manage Diabetes in India“, Huffpost, 2016
Jana Care, “AINA: Analytical Performance Summary”
Lavakare, Jyoti. “Jana Care: Sidhant Jena’s start-up develops lifestyle app to help manage ‘diabesity’ “, The Economic Time, 2014
Jana Care, “AINA: Introdution”
Jana Care, “AINA: User Manual“
Compliance with regulations
Aina's accuracy meets ISO15197:2013 regulatory standards needed for CE and US FDA approval.
Evaluation methods
Jana Care has conducted trials with Mass General Hospital, the National University Hospital, Xiangya Hospital, Changsha, China and others.
Other Information
The device also docks with the Aina Station, a point-of-care platform that can sit in a primary care office or clinic to screen patients with the goal of reduce time that patients have to wait for results from a central lab.

Agriculture
February 2, 2024
Implemented by
Gradian Health Systems

Agriculture
November 22, 2024
Implemented by
Appropriate Energy Saving Technologies Limited (AEST)

Agriculture
February 5, 2024
Implemented by
Centre for Vision in the Developing World

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 1, 2024
Implemented by
Infantrust Parenting Solutions

Agriculture
February 16, 2024
Implemented by
Equalize Health
Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
December 3, 2024
Implemented by
Veryday (McKinsey Design)

Agriculture
February 8, 2024
Implemented by
GE Healthcare
Have thoughts on how we can improve?
Give Us Feedback