Agriculture
March 8, 2024
Laerdal Servi Ventilator
Read SolutionImplemented by
Laerdal Global Health

Updated on November 22, 2023
·Created on May 20, 2016
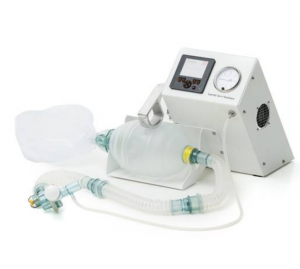
This is a manual resuscitator for infants.
The Laerdal Upright Newborn Bag-Mask is a self-inflating, manual, reusable resuscitator intended for newborns and infants with up to 10 kg body mass who require respiratory support. This product was selected for inclusion in the WHO’s 2014 Compendium of Innovative Health Technologies for Low-Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$100.00
Target Users (Target Impact Group)
Household, Community, Small and Medium-sized Enterprises
Distributors / Implementing Organizations
Laerdal Global Health, Laerdal Medical, Helping Babies Breathe (HBB) Development Alliance
Manufacturing/Building Method
Upright is manufactured at a plant in China. The product is produced as order requests are received.Interview with representative
Intellectural Property Type
Trademarked
User Provision Model
The Laerdal Upright Neonatal Bag-Mask can be purchased on a not-for profit basis for the 75 countries with the highest maternal and neonatal mortality rates. Those that do not qualify for not-for-profit purchase can order the product from Laerdal Medical. Partners in the Helping Babies Breathe Global Development Alliance are using Upright with the Helping Babies Breath resuscitation training curriculum. The products included in the training are typically left with providers upon completion of the program. The product has also been purchased by Ministries of Health.Interview with representative.
Distributions to Date Status
Over 5,000 units have been distributed as of May 2016. Upright is used in over 40 countries.Interview with representative
Design Specifications
Upright operates like a normal bag-valve-mask device except that the bag has a vertical stance over the mask. The mask fits with standard 15 mm inner-diameter conical connectors and attaches with a snap fit when pressed completely into place. Upright has a pressure release valve (“pop-off ”) which limits the airway pressure to 30-45 cmH2O and makes a hissing sound when opened. A higher pressure can be applied by pressing downwards on the valve with the index finger while squeezing the bag. Testing of lip valve function, pressure release valve, inlet-valve opening, and product sealing must be conducted before use. Procedure for each test are available in Directions for Use.
Expiratory resistance: <2.5 cm H20 at 5 LPM; Inspiratory resistance: <0.5cm H20 at 5 LPM; Tidal volume: > 150 mL; Dead space: 4 mL (water volume)
Operating temperature: -18-50°C; Storage temperature -40-60°C; External dimensions: 72mm x 85mm x 217mm; Mass: 190g
Sterilization method: boiling, chemical glutaraldehyde solution or steam autoclaving
Technical Support
A 1-year warranty is provided under the Laerdal Global Warranty . Laerdal Global Health recommends that customers contact them through their online customer feedback form or email if a product is malfunctioning.Interview with representative
If properly cared for, the product does not require any preventative maintenance. The product can be serviced by a general technician if it needs to be repaired. Depending on the nature of the repair, the manufacturer recommends to purchase a new unit if the bag-mask is not functioning properly because the product is low-cost.Interview with a representative
Replacement Components
All spare parts listed in the Directions for Use can be purchased from Laerdal Global Health via email. Supplementary oxygen kits can also be purchased on the Laerdal Global Health website for $4 USD. ^(Interview with representative)
Lifecycle
The device may last for years or decades, depending on how it is stored and reprocessed.Interview with representative
Manufacturer Specified Performance Parameters
Upright is suggested to make obtaining mask seal and providing effective ventilation easier than other newborn resuscitation equipment. The upright orientation of the mask is intended to help the user provide an even, downward pressure onto the mask. The mask has a thicker and broader top surface and a more pliable bottom cuff part to make it easier to hold the mask correctly and thus enabling a better mask seal.
Vetted Performance Status
Upright has undergone reprocessing and disinfection testing by NAMSA and passed all acceptance criteria. Upright was tested by PATH in Seattle where results support that the product improves ventilation, is easier to use, and decreases mask leak.1 A study conducted with 87 Tanzanian and Norwegian nursing and medical students revealed similar results.2 Clinical studies in Uttar Pradesh, India and Haydom, Tanzania were recently completed and publications will be available soon.Interview with representative. Future studies are set to take place in Nepal, Uganda, and India.
Safety
Laerdal Upright Newborn Bag-Mask is equipped with a pressure release valve ("pop-off") that limits the airway pressure to 30-45 cmH20. Instructions are provided in the Directions for Use in the event that the patient valve becomes contaminated with vomit.
Complementary Technical Systems
The Laerdal Newborn Mask is compatible with the NeoNatalie Resuscitator and other resuscitators. An oxygen kit is available as an optional accessory that can be ordered separately. A Positive End-Expiratory Pressure (PEEP) accessory is currently being developed for Upright and is anticipated to be available by the end of 2016.Interview with representative
Academic Research and References
Coffey, P.S., Saxon, E.A., Narayanan, I., DiBlasi, R.M. Performance and Acceptability of Two Self-Inflating Bag-Mask Neonatal Resuscitator Designs. Respiratory Care. Published: July 7, 2015. doi: 10.4187/respcare.03867Thallinger, M., Ersdal, H.D., Ombay, C., Eilevstjønn, J., Størdal, K. Randomised comparison of two neonatal resuscitation bags in manikin ventilation. Archives of Disease in Childhood- Fetal and Neonatal Edition. Published: October 5, 2015. doi:10.1136/archdischild-2015-308754
Compliance with regulations
Upright has received CE mark 0434. Meets ISO 10651-4:2002 (Lung ventilators- Particular requirements for operator-powered resuscitators). ANSI/AAMI/ISO 17665, AAMI TIR12,AAMI TIR 30 and ANSI/AAMI ST79 (all regarding reprocessing and disinfection).
Evaluation methods
Details of evaluations done both in the lab and in clinical settings can be found in the Upright Newborn Bag-Mask Report
Other Information
Additional awards and recognitions include: Inclusion in the WHO 2014 Compendium, Path’s Innovation Countdown 2030 Report, Norsk Design- Award of Design Excellence, Core 77 Design Awards, NPR feature as Top 5 innovation

Agriculture
March 8, 2024
Implemented by
Laerdal Global Health
Agriculture
February 26, 2024
Implemented by
Envo mask
Agriculture
September 27, 2024
Implemented by
Design That Matters (DtM)

Agriculture
March 9, 2024
Implemented by
Laerdal Global Health

Agriculture
January 17, 2024
Implemented by
Aquagenx

Agriculture
September 27, 2024
Implemented by
Teheca Limited

Agriculture
February 29, 2024

Agriculture
February 5, 2024
Implemented by
Vestergaard

Agriculture
December 19, 2023
Implemented by
Aqua-Aero WaterSystems BV (AAWS)

Agriculture
September 27, 2024
Implemented by
MamaOpe
Have thoughts on how we can improve?
Give Us Feedback
In categories where it seems like there are discrete options to choose from, for example “pricing structure”, I think it would be nice for the reader to know what all of the potential options are.
For “Compliance with regulations in target market”, I think more information than yes/no is important. For example, “Yes – CE Mark” would be more informative.
The category labeling “ventilators” is confusing to me for the first 3 products. Could it be something more general, like “respiratory support”?
The effort and rigor in the design and manufacturing process to secure regulatory approval should not be underestimated. This requires documentation starting at the initial design and concept stages of the product. For example, is there a documented Failure Mode and Effects Analysis to guide the design process that defines both expected and reasonably unexpected misuse of the product – and the associated design, process control and/or (lastly and least effectively) labeling to effectively mitigate these risks?
Manufacturing of this type of product requires documented process and assembly standards. In volumes of 100 special attention needs to be paid to ensure that each unit is not a unique “one of†but rather follows and industrialized, documented and controlled assembly
The effort and rigor in the design and manufacturing process to secure regulatory approval should not be underestimated. This requires documentation starting at the initial design and concept stages of the product. For example, is there a documented Failure Mode and Effects Analysis to guide the design process that defines both expected and reasonably unexpected misuse of the product – and the associated design, process control and/or (lastly and least effectively) labeling to effectively mitigate these risks?