Agriculture
November 17, 2023
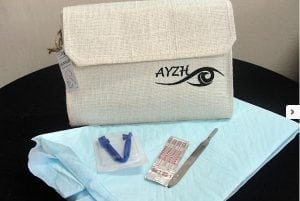
Janma Clean Birth Kit
Read SolutionImplemented by
AYZH
Updated on February 15, 2024
·Created on August 1, 2019
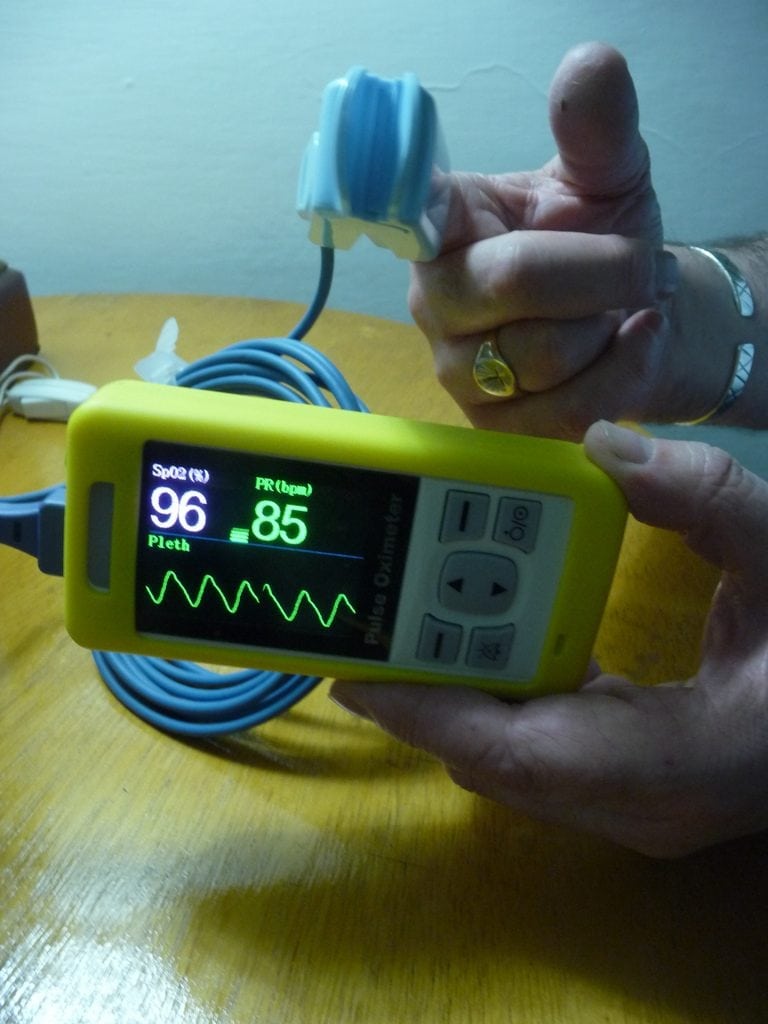
The Lifebox Pulse Oximeter is a device for monitoring oxygen saturation in low-resources settings.
The Lifebox Pulse Oximeter is a rechargeable monitor for oxygen saturation that can be used for adults and children. It is especially designed for use in low-resource settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$250.00
Target Users (Target Impact Group)
Household, Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
Lifebox, Mercy Ships, Smile Train are building partnerships with the manufacturer to distribute the pulse oximeter.
Regions
Worldwide
Manufacturing/Building Method
The pulse oximeter is manufactured by Acare Technology Co. Ltd. which specializes in monitoring and anesthesia devices.
Intellectural Property Type
Select Type
User Provision Model
The pulse oximeter can be purchased directly from the manufacturer and is also distributed by partner NGOs (including Mercy Ships and Smile Train), national anesthesia societies and local facilities.
Distributions to Date Status
As of July, 2021, Lifebox has distributed 28,000 pulse oximeters in over 116 countries across the globe.
Consumables
probes and batteries
Detection sensitivity
+/- 2%
Indispensable equipment for function (Y/N)
N
Maintenance or calibration required by user at time of use? (Y/N)
N
Number of Tests Performed
2
Power supply type: Continuous, Recharging only (V, time required, battery life), Other
Continuous, Recharging with the battery runtime of 14 hours
Time required for procedure (minutes)
Continuous monitoring
Design Specifications
The Lifebox Pulse Oximeter is designed for settings with inconsistent electrical power, as the charger protects the device from surges and the battery(3 AA Alkaline batteries or Lithium ion rechargeable battery) has a life of 14 hours in case of a power outage. It can be paired with two probes: a universal probe for 3+ months and a pediatric probe, both of which are included with the device purchase.
The product has the following specifications:
Pulse rate range: 25 - 250 bpm
Resolution : 1 bpm
It has a display type 2.4” color display 320 x 240 pixels with the following parameters on display; Digital SpO2, Pulse Rate, Pleth bar & SpO2 waveform
Technical Support
The first line of technical support is the instructional video, user manual, and troubleshooting guide.
Additional support can be found by contacting the manufacturer.
Replacement Components
The product comes in a package that includes:
These components can be replaced but the internal components of the oximeter cannot.
Lifecycle
2+ years for the oximeter, 1 year for the probes
Sterilization (with a cloth and disinfectant) is required and described in the user manual. Some maintenance is also required. Instructions for both are provided in the user manual.
Manufacturer Specified Performance Parameters
Lifebox says their pulse oximeter
Vetted Performance Status
There are no performance-specific accuracy data on this instrument. The accuracy of the Lifebox pulse oximeter is comparable to FDA-approved pulse oximeters designed for high income countries. The Lifebox oximeter was found to meet USA FDA 510(k) standards for the detection of hypoxia.
Safety
If surges more severe than EN61000-4-5 +- 1KV line to line or +- 2KV line to ground are likely to occur, a surge protector should be used when charging the oximeter.
Complementary Technical Systems
None
Academic Research and References
Dubowitz G, Breyer K, Lipnick M, Sall JW, Feiner J, Ikeda K, Macleod DB, Bickler PE. Accuracy of the Lifebox pulse oximeter during hypoxia in healthy volunteers. Anaesthesia. 2013;68(12):1220–1223.
Finch LC, Kim RY, Ttendo S, Kiwanuka JK, Walker IA, Wilson IH, Weiser TG, Berry WR, Gawande AA. Evaluation of a large-scale donation of Lifebox pulse oximeters to non-physician anaesthetists in Uganda. Anaesthesia. 2014;69(5):445–451.
Albert V, Mndolo S, Harrison EM, Osullivan E, Wilson IH, Walker IA. Lifebox pulse oximeter implementation in Malawi: evaluation of educational outcomes and impact on oxygen desaturation episodes during anaesthesia. Anaesthesia. 2017;72(6):686–693.
Sama HD, Maman AFOBN, Walker IA. Incidence of hypoxia and related events detected by pulse oximeters provided by the Lifebox Foundation in the maternity unit at Sylvanus Olympio University Teaching Hospital, Togo. Journal of Anesthesia. 2015;29(6):971–973.
Desalu I, Diakparomre OI, Salami AO, Abiola AO. The effect of nail polish and acrylic nails on pulse oximetry reading using the Lifebox oximeter in Nigeria. The Nigerian Postgraduate Medical Journal. 2013;20(4).
Enright A, Merry A, Walker I, Wilson I. Lifebox: A Global Patient Safety Initiative. A & A Case Reports. 2016;6(12):366–369.
Lifebox. “FDA-standard for the low-resource setting“, 2013
Compliance with regulations
The Lifebox Pulse oximeter complies with the World Health Organization and World Federation of Societies of Anesthesiologists standards for suitable application in under-resourced environments.
Evaluation methods
The accuracy of the Lifebox pulse oximeter was compared against arterial hemoglobin oxygen saturation by academic researchers. It was also compared in accuracy to FDA-approved pulse oximeters.
Other Information
None

Agriculture
November 17, 2023
Implemented by
AYZH

Agriculture
December 29, 2023
Implemented by
LifeStraw

Agriculture
December 16, 2024
Implemented by
MPOWERD

Agriculture
February 29, 2024
Implemented by
Motivation

Agriculture
December 29, 2023
Implemented by
Sawyer
Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360

Agriculture
December 19, 2023
Implemented by
RealRelief
Agriculture
June 26, 2024
Implemented by
SweetSense Inc

Agriculture
December 7, 2023
Implemented by
Solar Ear

Agriculture
February 20, 2024
Implemented by
Sterilux
Have thoughts on how we can improve?
Give Us Feedback
It is important to consider the battery life per charge cycle as resource constrained environments are constantly handling high volumes of patients and limited access to medical devices and staff. It would be ideal for the battery to last up to 8-12 hours or more to account for extended usage in these environments. It is also important to consider whether preventative maintenance is required throughout the 2 year lifetime of the product and the level of training that would be required for technical staff to perform on-site repairs. Additionally, if the probes are intended to be reusable than they must be able to be able to withstand cleaning methods used in the target settings.
Academic literature and evaluation is extensive for a product designed for use in resource constrained clinic environments.
Additionally, this product requires a reliable source of power.