
Agriculture
December 27, 2023
AquaFilter Family
Read SolutionImplemented by
Aquabox, UK
Updated on September 26, 2024
·Created on July 8, 2016
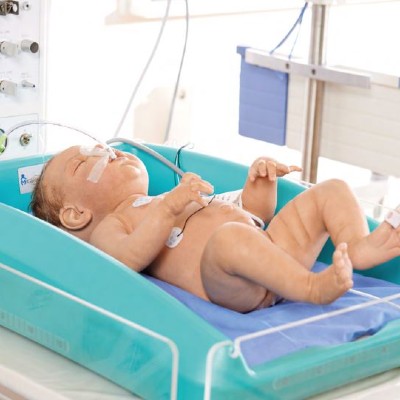
An affordable cooling device for treating birth asphyxia.
MiraCradle™ – Neonate Cooler is a passive cooling device which uses savE® phase change material (PCM) technology to induce therapeutic hypothermia among newborns suffering from birth asphyxia.
Target Regions
Worldwide
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$2,258.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises
Distributors / Implementing Organizations
The product is currently being distributed worldwide through the VIAGlobalHealth, Colmed and Dräger websites.
Regions
Worldwide
Manufacturing/Building Method
MiraCradle's materials are manufactured and mass produced by Pluss Polymers.
Intellectural Property Type
Select Type
User Provision Model
MiraCradle can be purchased online through multiple platforms such as VIAGlobalHealth, Dräger and Colmed.
Distributions to Date Status
MiraCradle is present in 165 hospitals across the globe as of August 2021.
Duration target temperature maintained (Hours)
72 hours
Accuracy range of target temperature (°C)
33-34°C
Power supply type
Phase Change Material cooling. No electricity needed. However, PCMs can be pre-cooled and stored for use when power is available.
Consumables
None
Indispensable equipment for function (Y / N)
Domestic refrigerator and temperature monitoring equipment
Maintenance or calibration required by user at time of use? (Y/N)
No
Design Specifications
The MiraCradle operates with the use of phase change materials (PCM) designed to melt/freeze at the temperatures which would ensure a temperature regulation of the infant between 33 and 34°C for up to 72 hours. The PCM absorbs heat from the baby thereby cooling the baby. The device uses two PCMs which have a melting/freezing temperature of 29°C and 21°C and an insulated cradle to place these PCMs. A gel bed is placed above the PCMs to give a comfortable surface for the baby
Operational requirements as noted in the 2014 WHO Compendium of Innovative Health Technologies for Low-Resource Settings:
Pre-cool the PCMs FS-29 and FS-21 in a refrigerator for a period of 6-8 hours. Place the 3 pre-cooled PCM FS-29 slabs in the insulated cradle. On top, place 2 slabs of PCM FS-21. Place the gel bed above the PCMs. Place the complete set in the bassinet of a standard infant warmer. Place the baby over the gel bed. Use a rectal probe to measure the temperature continuously. If the temperature decrease below 33°C, use a warmer to increase the temperature.
Device Weight: 13 kg; Dimensions: 620 mm x 460 mm
Technical Support
MiraCradle's website section on maintenance and operation suggests that the product is easy to use and should require zero maintenance. However, they also provide a detailed user manual, training video, and two guides for assembly and temperature monitoring, along with contact information for any queries.
Replacement Components
Instructions in the user manual suggest as follows: Replace savE FS-29 PCM and savE FS-21 PCM after they have individually been used 500 times. Do not use either after their expiry dates under any circumstances. Replace Conduction Mattress one year from the date of first use. Do not use Conduction Mattress after expiry date under any circumstances. Replace the temperature indicator on savE FS-29 one year from the date of first use.
Lifecycle
3 years, as listed in the 2014 World Health Organization's Compendium of Innovative Health Technologies for Low-Resource Settings.
Manufacturer Specified Performance Parameters
Non-electric and able to maintain temp of 33.5 °C for a period of 72 hoursInterview with representative
Vetted Performance Status
Neonatologist Niranjan Thomas first began experimenting with and testing gel packs as a cooling treatment nearly a decade ago (relative to Spring 2016) at the Christian Medical College in Vellore, India. The MiraCradle team also beta-tested the product with hospitals in India. A randomised controlled trial was performed in India, finding that MiraCradle's therapeutic induced hypothermia reduces brain injury rates at the NICU.
Safety
Safety instructions are listed in the user manual , such as reviewing the manual and warning/caution labels carefully before use, avoidance of use of damaged contents and contact for replacement, and storage of parts as per instructions. Additionally, ensure that sufficient training is required and that parts are sanitized before use.
Complementary Technical Systems
A domestic refrigerator is required for freezing the PCM; the temperature of NICU should be maintained above 25°C. Also requires equipment for monitoring the vital parameters of the newborn during treatment (ex. rectal probe)
Academic Research and References
Catherine, C., Bhat V., Adhisivam, B., Palanivel, C., 2021, Effect of Therapeutic Hypothermia on the Outcome in Term Neonates with Hypoxic Ischemic Encephalopathy—A Randomized Controlled Trial, Journal of Tropical Pediatrics, 67(1).
Aker, K., Støen, R., Thomas, N., 2020, Therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy in India (THIN study): a randomised controlled trial, Archives of disease in childhood, 105(4), pp. 405–411.
Prashantha Y.N., Suman Rao P.N., Shashidhar A., 2019, Therapeutic hypothermia for moderate and severe hypoxic ischaemic encephalopathy in newborns using low-cost devices – ice packs and phase changing material, Paediatrics and International Child Health, 39(4), pp. 234-239.
Thomas N., Abiramalatha T., Prashantha YN., Nimbalkar S., 2018, Phase Changing Material for Therapeutic Hypothermia in Neonates with Hypoxic Ischemic Encephalopathy – A Multi-centric Study, Indian Pediatrics, 55(3), pp. 201-205.
Thomas, N., Chakrapani, Y., Rebekah, G., Kareti, K., Devasahaym, S., 2015, Phase Changing Material: An Alternative Method for Cooling Babies with Hypoxic Ischaemic Encephalopathy, Neonatology, 107, pp. 266-270.
A list of highlighted publications are located on the company website.
Compliance with regulations
CE certification and ISO 13485
Other Information
The company obtained a partnership with Fanem Medical Devices in April, 2021, in order to expand its distribution capacity throughout India.

Agriculture
December 27, 2023
Implemented by
Aquabox, UK

Agriculture
December 18, 2023
Implemented by
Be Girl

Agriculture
January 11, 2024
Implemented by
Kenya Ceramic Project (KCP)

Agriculture
January 8, 2024
Implemented by
DayOne Response
Agriculture
December 19, 2023
Implemented by
Hadleigh Health Technologies and Rice University

Agriculture
February 16, 2024
Implemented by
Eniware
Agriculture
February 8, 2024
Implemented by
Design That Matters (DtM)

Agriculture
January 10, 2024
Implemented by
NativeEnergy
Agriculture
December 1, 2023
Implemented by
SMS Medical College Hospital (Ram Chandra Sharma with the team of doctors)

Agriculture
January 2, 2024
Implemented by
Klorfasil
Have thoughts on how we can improve?
Give Us Feedback
The Sistema Biobolsa BB40 is a bigger flexible plastic biogas system, mainly directed at famers with dairy cattle, who can use the gas to run engines for electricity generation. The company make a range of biogas plant sizes. The material of the flexible bags seems to be PVC plastic, which has a longer lifetime, but is more expensive than lower quality materials. As with all flexible plastic systems, it needs to be looked after to prevent the plastic being punctured. Patches can be glued over holes in a PVC material.
Steve Harston says:
Under the section “Distribution to date” it would be nice if there was a date associated with the comment. For example, the text currently says that the MiraCradle is in 88 hospitals but when was this information last updated?
I’d change the description a little to explain better about what it is. Here’s a re-write: The G110 is a three-wheeled farm truck that hauls goods and doubles as a power source for attachable tools. The truck provides power and transportation to small-holder farmers and agricultural businesses that would otherwise not be possible. Apart from transport, the G110 also power attachments such as water pumps, maize grinders, generators, threshers and planters, and it can pull a plow. In areas where people carry water jugs and sacks of produce on their heads between farms, homes and markets, the G110 provides hauling capacity that saves time and, ultimately, money.
Name changed from MAPS G110 to AgRover