
Agriculture
December 27, 2023
AquaFilter Family
Read SolutionImplemented by
Aquabox, UK
Updated on March 9, 2024
·Created on August 31, 2021
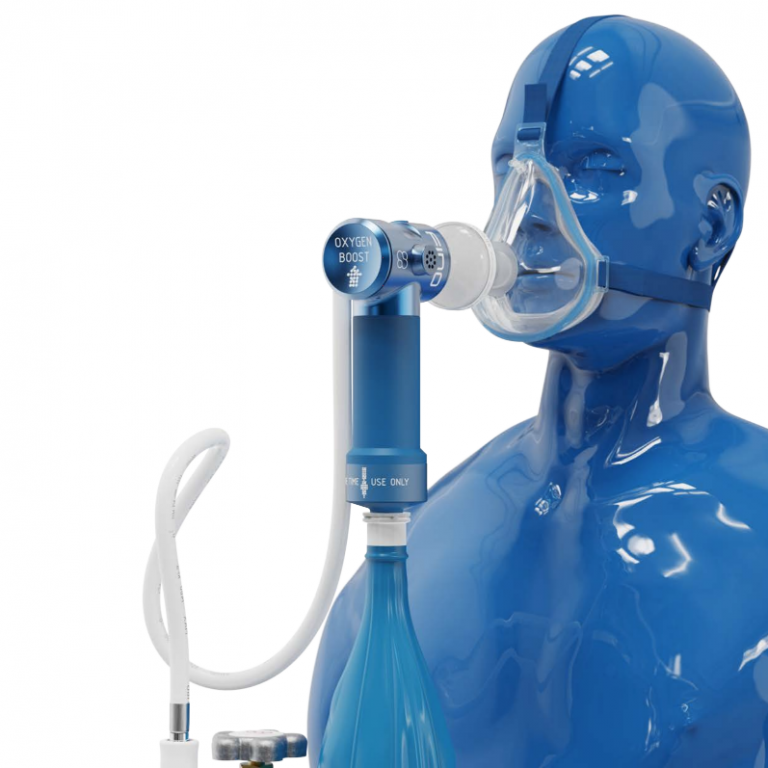
FIDO is a portable oxygen system designed to help patients breath outside of clinical settings. Courtesy of WHO Compendium 2021
FIDO by Mirola is a portable, mechanical rebreathing system that enables a patient to increase their oxygen intake without requiring medical expertise. A canister of oxygen is connected to an intake filter and face mask.
This product was selected for inclusion in WHO’s 2021 Compendium of Innovative Health Technologies for Low‐Resource Settings.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$2,700.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
In September 2020, the Swedish Rescue Service ordered the first batch of FIDO products, which they plan to incorporate into their rescue vehicles.
Competitive Landscape
Direct competitors include Solar-Powered Oxygen Delivery (SPO2) and O2 Cube.
Regions
Europe
Manufacturing/Building Method
This product is manufactured in the Stockholm region and Gnosjö in Sweden.
Intellectural Property Type
Patent
User Provision Model
This product is purchased by health systems to distribute for patient use when necessary.
Distributions to Date Status
Unknown
Consumables
Oxygen tank
Power supply type
None
Indispensable equipment for function
Yes, a face mask
Maintenance or calibration required by user at time of use? (Y/N)
Yes
Display Type
None
Gas Flow Range (L/min)
2-16 L/min
Pressure Range (cm H2O)
4079-5098 cm H2O (4-5 bar)
Patient population (specify)
Adults
Design Specifications
A patient wears FIDO over their mouth and nose. When they exhale, air is filtered through a limestone cartridge, which collects most of the CO2. An attached balloon collects and recirculates the exhaled air, mixing it with oxygen from the attached canister. When the patient inhales, the air is once again passed through the limestone cartridge.
Technical Support
Support is provided by the manufacturers.
Replacement Components
Replacement components are available from the manufacturer.
Lifecycle
15-20 year estimated lifetime
Manufacturer Specified Performance Parameters
This device is designed to increase the concentration of oxygen inhaled by a patient.
Vetted Performance Status
This product was vetted and inspected for quality assurance required for mobile oxygen devices by the Research Institutes of Sweden (RISE).
Safety
The device includes a safety valve that opens to allow the patient to keep breathing surrounding air if the oxygen supply starts to fail.
Complementary Technical Systems
None
Academic Research and References
None
Mirola, “Swedish Mirola Rescue has received its first order from the Swedish Rescue Services“, 2020
Research Institutes of Sweden, “EC CERTIFICATE – Mirola Rescue AB“, 2020
Compliance with regulations
In March 2020, this product earned the EC Certificate by the Research Institutes of Sweden.
Evaluation methods
Laboratory testing and certification for mobile oxygen devices.
Other Information
None

Agriculture
December 27, 2023
Implemented by
Aquabox, UK

Agriculture
December 18, 2023
Implemented by
Be Girl

Agriculture
January 11, 2024
Implemented by
Kenya Ceramic Project (KCP)

Agriculture
January 8, 2024
Implemented by
DayOne Response
Agriculture
December 19, 2023
Implemented by
Hadleigh Health Technologies and Rice University

Agriculture
February 16, 2024
Implemented by
Eniware

Agriculture
December 19, 2023
Implemented by
GlobalNeoNat
Agriculture
November 17, 2023
Implemented by
AYZH

Agriculture
December 29, 2023
Implemented by
LifeStraw

Agriculture
December 16, 2024
Implemented by
MPOWERD
Have thoughts on how we can improve?
Give Us Feedback