
Agriculture
March 9, 2024
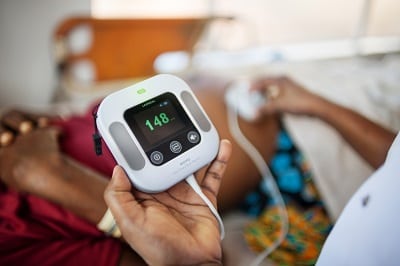
NeoBeat Newborn Heart Rate Meter
Read SolutionImplemented by
Laerdal Global Health
Updated on September 26, 2024
·Created on July 8, 2016
Moyo is a fetal heart rate monitor designed for low resource settings.
Moyo is a fetal heart rate (FHR) monitor designed for intermittent and prolonged monitoring in low-resource settings. The devices is intended to enable birth attendants to make appropriate obstetric interventions without interrupting existing routines.
Information for this product was provided courtesy of the WHO’s 2016 Call for Innovative Health Technologies for Low-Resource Settings.
Target Regions
Worldwide
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$198.00
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises
Distributors / Implementing Organizations
Laerdal Global Health
Regions
Worldwide
Manufacturing/Building Method
Moyo is currently manufactured in China in batches (Per Interview with representative)
Intellectural Property Type
Select Type
User Provision Model
Moyo can be purchased on the Laerdal Global Health website at a subsidized price of $198 with the online order form.
Distributions to Date Status
739 devices have been sold and 100+ have been donated by Laerdal as of August 2017. Interview with representative
Power Supply Type
Internal rechargeable battery, Lithium-ion, 3.7 V, 2600 mAh
Consumables
Water-based ultrasound gel or water (for short term monitoring)
Indispensable equipment for function (Y/N)
N
Maintenance or calibration required by user at time of use?
N
Display Type
LCD display, LED indications, and audio alarm (72 dB)
Gestation Required for Heart Rate Detection (weeks)
Moyo FHR was designed for term women, but it can also be used during the antenatal period. One study shows that it can detect at least 19 weeks gestational age. Interview with representative
Material of Construction
ABC/PC, stainless steel, ABS (ultrasound transducer), non-latex high elastic polyester (abdominal belt), non-latex polyester (neck strap)
Design Specifications
Moyo is designed for continuous and intermittent fetal heart rate monitoring. The device uses a 9-crystal Doppler ultrasound sensor that enables detection of the fetal heart rate in less than 5 seconds. The detection area is 900 cm2 which makes palpating and aiming for the heart less critical. The device is also equipped with dry electrodes which allow the health care provider to easily compare maternal pulse with FHR. The default display for Moyo includes the present heart rate measurement, battery level, and Doppler volume level. The user interface also includes a history graph feature to review measurements obtained during the last 30 minutes.
Moyo includes an audiovisual alarm that is triggered by lost FHR (no FHR or <50 bpm lasting for 60 seconds), abnormal FHR (<100 bpm or >180 bpm lasting for more than 3 minutes), or low battery. The device contains an internal rechargeable battery that has a run time of 10 hours and takes 5 hours to recharge. The device draws .74 W when in use.
The probe has a frequency of 1MHz and an intensity of <5 mW/sq. cm. The thermal index (TI) and mechanical index (MI) of the probe are always below 1.0. Moyo uses a proprietary software that is language independent and allows for the configuration of some settings by user.
The device includes a neck strap that is designed to enable mobility of the mother during continuous monitoring. The device also includes an belt to attach the transducer to the abdomen.
Dimensions: 96 x 96 x 24 mm; Weight: 300 g; Operating temperature: 0-40 °C; Storage/shipping temperature: -30-70 °C Storage/shipping air pressure: 550-1060 hPa; Operating/storage/shipping humidity: Up to 95% RH, non-condensing Ingress protection: IP41
The device and its accessories can be cleaned or disinfected with either a soapy water, 70% ethanol solution, or bleach solution. Instructions for cleaning and disinfection procedures for each component are found in the Directions for Use. Moyo is to be disposed of in an appropriate recycling facility in accordance with local regulations.
Technical Support
There are no serviceable parts in the system.
A 1-year warranty is provided under the Laerdal Global Warranty . Laerdal Global Health recommends that customers contact them through their online customer feedback form or email if a product is malfunctioning.Interview with representative.
Troubleshooting guidance is available for the user in the Directions for Use. Laerdal Global Health shares the materials for an evidence-based training program that is being tested in Tanzania to implementing partners upon request.Interview with representativeJhpiego and ACOG are currently developing a training program for Complicated Labour and Birth which will deal with issues around prolonged labour and abnormal fetal heart rate.Interview with representative
Replacement Components
Moyo does not have any replaceable parts, including the battery. Accessories for the device (abdominal transducer belt, neck strap, and battery charger and cable) can be independently replaced.
Lifecycle
The estimated lifetime and shelf life is 2-5 years.
Manufacturer Specified Performance Parameters
The ultrasound transducer can detect heart rate within the range of 50-250 bpm with short term average accuracy of ±5 bpm in the range of 50-200 bpm. The maternal heart rate electrodes have a display range of 30-250 bpm with short term average accuracy of ±5% or ±5 bpm, whichever is greater, in the range 50 – 150 bpm.
Vetted Performance Status
Randomized control trials are being conducted in two referral hospitals in Tanzania. One study is comparing Moyo to Doppler and the other is comparing Moyo to hand-held fetoscopes. A parallel study is being conducted by Laerdal Global Health's implementing partners in Tanzania that aims to demonstrate the efficacy of Moyo when coupled with an evidence-based training program. Moyo is also being used to monitor fetal heart rate in a clinical study in Nepal that is investigating the effect of timing of cord clamping in newborns. Moyo will also be used in a study on the indicator for stillbirth.Interview with representative Results: Continuous Moyo compared to intermittent Pinard in Haydom or Freeplay Doppler in Muhimbili was shown to have a higher abnormal FHR detection rate. When given the option to choose among Moyo and the other available FHR monitoring devices, almost 90% of health care providers chose to monitor using Moyo in Dongobesh. Use of Moyo was associated with improved monitoring and documentation. In Temeke, the use of Moyo decreased rate of lack of documentation and showed a significant increase in frequency of monitoring. Interview with representative
Safety
Precautions and warnings for Moyo are listed in the Directions for Use.
Complementary Technical Systems
A reliable power source must be used to recharge the product battery.
Academic Research and References
Lafontan SR, Sundby J, Ersdal H, Abeid M, Kidanto H, Mbekenga C. “I Was Relieved to Know That My Baby Was Safe”: Women’s Attitudes and Perceptions on Using a New Electronic Fetal Heart Rate Monitor during Labor in Tanzania. International Journal of Environmental Research and Public Health. 2018;15(2):302.
Compliance with regulations
CE marked (class II medical device), ISO 13485, EN 1789:2007 +A1:2010, EN ISO 10993-1:2009 / AC:2010, EN ISO 14971:2012, EN 15223:2012, EN 60601-1:2006 + A1:2012, EN 60601-1-2:2007 / AC:2010, EN 60601-1-6:2010, EN 60601-1-8:2007 /A1:2013, EN 60601-2-37:2008/A11:2011, EN 62133:2013, EN 62304:2006 / AC:2008
Other Information
Moyo is a recipient of:

Agriculture
March 9, 2024
Implemented by
Laerdal Global Health

Agriculture
February 5, 2024
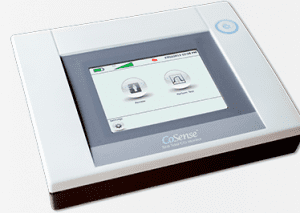
Implemented by
Shift Labs
Agriculture
March 11, 2024
Implemented by
Capnia
Agriculture
September 27, 2024
Implemented by
Neopenda

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief

Agriculture
December 31, 2023
Implemented by
Potential Energy

Agriculture
June 22, 2024
Implemented by
World Bicycle Relief

Agriculture
June 8, 2024
Implemented by
ClickMedix
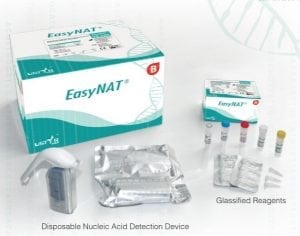
Agriculture
February 14, 2024
Implemented by
USTAR Biotechnologies (Hangzhou) LTD
Have thoughts on how we can improve?
Give Us Feedback