Agriculture
September 27, 2024
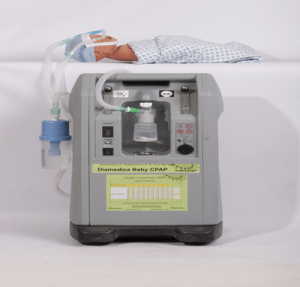
Diamedica Baby CPAP
Read SolutionImplemented by
Diamedica
Updated on November 24, 2023
·Created on September 6, 2016
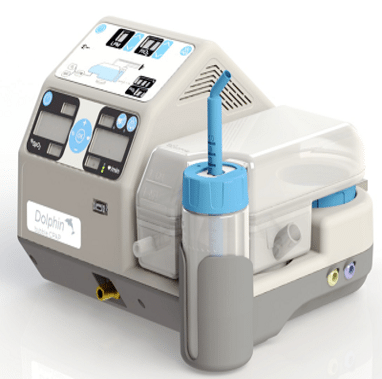
Dolphin CPAP is a prototype that provides bubble continuous positive air pressure (CPAP) therapy to support the airways of pre-term and low-birth weight newborns and incorporates Pulse Oximetry.
Dolphin CPAP is a prototype that provides bubble continuous positive air pressure (CPAP) therapy to support the airways of pre-term and low-birth weight newborns and incorporates Pulse Oximetry. The device mixes air with oxygen, adds humidity to the gas mixture, and then delivers it to the patient at a controlled flow rate.
Target Regions
Africa, Central Asia, North Asia, South Asia, Southeast Asia
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises
Distributors / Implementing Organizations
Medical Technology Transfer and Services, national and international distributors, Thrive Networks
Competitive Landscape
Direct competitors include Pumani Bubble Continuous Positive Airway Pressure (bCPAP) Device.
Regions
Africa, Central Asia, North Asia, South Asia, Southeast Asia
Manufacturing/Building Method
The device is manufactured by MTTS in Hanoi, Vietnam. Interview with representative
Intellectural Property Type
Other
User Provision Model
MTTS has a public-private partnership with Thrive Health’s Newborn Health Program (formerly Breath of Life). Through the Newborn Health Program, Thrive Health and MTTS have partnered with Ministries of Health, national universities, and leading neonatologists across 14 countries to implement sustainable training programs and appropriate medical equipment in low-resource environments to reduce neonatal mortality and morbidity.
The device will also be available through the MTTS network of national and international distributors. The device can also be ordered directly from MTTS if there is no local presence. The Dolphin CPAP will be sold to hospitals, NGOs, and local and central governments all over the world.Interview with representative
Distributions to Date Status
Prototype. Not available on the market as yet. The device is anticipated to reach market in May 2017.
Design Specifications
The Dolphin CPAP is an all-in-one CPAP treatment and monitoring device. The design integrates gas mixing, humidification, bubble PEEP/CPAP chamber, air compressor and pulse oximiter components in one unit.
The gas mixer electronically blends FiO2. The humidification component is a passover humidifier that automatically refills for up to 3 days of continuous operation. The humidifier takes less than 20 minutes to warm up at room temperature. The integrated diaphragm air compressor eliminates the need for an external air source. Additional performance specifications for each component of the Dolphin CPAP are on the product webpage.
The Dolphin CPAP is 100% reusable. Most components of the patient circuit can be autoclaved or chemically disinfected if an autoclave is not available.
Unit dimensions (excl. support arm): 38 cm x 38 cm x 80 cm
Shipping (boxed) dimensions: 48 cm x 48 cm x 90 cm
Net Weight (with air compressor): 15 kg
Shipping Weight (with air compressor): 20 kg
Operating ambient temp: +19°C to +37°C
Operating humidity: 0% to 90% RH non condensing
Operating atmospheric pressure: 70-106kPa
Transport and storage ambient temp: +10°C to +85°C
Transport and storage humidity: 0% to 90% RH non condensing
Transport and storage atmospheric pressure: 70-106kPa
Technical Support
The serviceable parts in the Dolphin CPAP are subject to at least a 1 year warranty. A list of serviceable parts and the duration of the warranty per part are available on the product webpage.
Replacement Components
Serviceable parts can be repaired or replaced by the manufacturer if the part is under warranty. Accessories for the Dolphin CPAP (air compressor, battery pack, gas hose, and mains power cable) can be ordered separately.
Lifecycle
Unknown.
Manufacturer Specified Performance Parameters
Unknown.
Vetted Performance Status
A 12-month pilot program was completed for the MTTS CPAP, the predicate device to the Dolphin CPAP. Results of the pilot program with the predicate device indicated that 24-hour mortality from respiratory distress syndrome was reduced by 75% after the introduction of the device.1 Additionally, a study with 100 infants that compared the effects of MTTS CPAP to an industry standard device showed no significant difference between the outcomes. Vetted performance conducted for the Dolphin CPAP is unknown.
Safety
The Dolphin CPAP is equipped with over voltage / over current protection and a mean time between failure (MTBF) of 100,000 hours.
Complementary Technical Systems
Unknown.
Academic Research and References
Compliance with regulations
EN ISO 13485:2012 (certified quality management system), EN 60601-1:2006/AC2010, EN 60601-1-2:2007/AC2010, EN 60601-1-6: 2010, EN 60601-1-8:2007/AC:2010, EN 60601-2-23: 2000, EN 80601-2-61: 2011, EN 12342:1999/A1:2009, ISO 5356-1:2004, ISO 8185:2007, ISO 10993-5, ANSI/AAMI/EN 60601-1, UL/TUV, Energy star efficiency level V compliant, RoHS 2 compliant
Evaluation methods
Unknown
Other Information
Unknown

Agriculture
September 27, 2024
Implemented by
Diamedica
Agriculture
January 25, 2024
Implemented by
Waterologies

Agriculture
January 2, 2024
Implemented by
Uzima

Agriculture
February 23, 2024
Implemented by
Mobile ODT

Agriculture
January 27, 2024
Implemented by
Water and Environmental Engineering, University of Bristol

Agriculture
January 28, 2024
Implemented by
Energryn

Agriculture
March 8, 2024

Agriculture
March 8, 2024
Implemented by
Mechanical Ventilator Milano

Agriculture
February 26, 2024
Implemented by
Hemex Health
Agriculture
February 27, 2024
Implemented by
Mbarara University of Science and Technology
Have thoughts on how we can improve?
Give Us Feedback