Agriculture
March 11, 2024
CoSense ETCO Monitor
Read SolutionImplemented by
Capnia
Updated on September 27, 2024
·Created on June 23, 2016
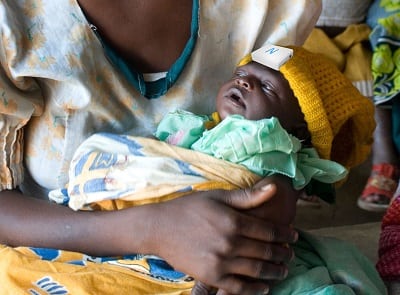
Neopenda is an infant hat with sensors to monitor vitals.
neoGuard is a simple, low-cost, low-power, sensor integrated into a baby hat that measures newborns’ key vital signs. Continuous monitoring improves the speed of action in life-threatening situations for neonates and ensures that when they are in distress, they have the best chance to receive the care they need to survive and thrive. The device is a prototype that is still in the early testing stages. Interview with representative
Target Regions
Africa
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$75.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises
Distributors / Implementing Organizations
Neopenda, Ugandan Pediatric Association
Competitive Landscape
Direct competitors include Lifebox Pulse Oximeter.
Regions
Africa
Manufacturing/Building Method
The devices are currently manufactured in-house and with local partners by Neopenda. Interview with representative
Intellectural Property Type
Select Type
User Provision Model
Ultimately the product may be purchased and distributed through wholesale distributors of medical equipment; in Uganda, the primary distributors are National Medical Stores for public hospitals and Joint Medical Store for private hospitals. We are also pursuing implementation with multi-national NGO partners. Interview with representative
Distributions to Date Status
This is a prototype and not available on the market as yet.
Alert mechanism
LED indicators and visual/auditory alarms at the central monitor Interview with representative
Clinical measurements monitored
Heart rate, respiratory rate, blood oxygen saturation, and temperature
Patient population
Newborns
Maximum duration of consecutive usage
Several days Interview with representative
Design Specifications
The Neopenda vital signs monitor is a wearable sensor device that gives voice to newborns in medical distress and enables more responsive, efficient medical care in low-resource hospitals. The device attaches to a baby hat or band and uses reflectance pulse oximetry and temperature sensors to continuously measure pulse rate, respiratory rate, blood oxygen saturation, and temperature. The vital signs from multiple newborns wearing the devices are wirelessly transmitted to a single tablet via Bluetooth Low Energy, where attending healthcare personnel can view all the patients’ health status in a centralized user-friendly interface. The interface generates plots with historical trend data, offers customizable alarm thresholds, and alerts staff of abnormal vital signs with visual and auditory alarms in real time. The devices are rechargeable and run for several days, so that newborns can wear them during their entire stay in the hospital. The simple, rugged design is built for the power, cost, and environmental challenges of low-resource facilities, and can be easily sanitized with alcohol wipes for reuse. The out-of-the-box system is not reliant on continuous power or wireless connectivity.Interview with representative
Target specifications for Neopenda Vital Signs Monitor. The device is presently in a testing phase but aims to achieve these targets.
Technical Support
The type and frequency of preventative maintenance needed is unknown. Neopenda is working with in-country engineers while refining the prototype to ensure that repairs can be performed on-site. Interview with representative
Replacement Components
Unknown
Lifecycle
The anticipated lifetime is 2 years.
Manufacturer Specified Performance Parameters
The Neopenda vital signs monitor gives voice to newborns in medical distress and enables responsive medical care in low-resource hospitals. The prototype is rapidly being refined and tested. Technical data is anticipated to be available in the coming months.
Vetted Performance Status
Neopenda is rolling out a pilot in Kampala, Uganda, in 2017 to assess the accuracy of the technology, and preparing for feasibility studies in up to six other facilities in four countries. Interview with representative
Safety
Unknown
Complementary Technical Systems
None
Academic Research and References
None
Compliance with regulations
The Neopenda device is enclosed in a case made of FDA compliant medical grade polymer, and uses 100% BPA-free, PCV-free, and phthalate-free materials. It has passed preliminary safety testing for compliance with specified standards of IEC 60601-1 Edition 3.1 (pertaining to medical electrical equipment safety), ISO 80601-2-56 (pertaining to clinical thermometers), and ISO 80601-2-61 (pertaining to pulse oximeter equipment) by a third party in February 2017. Interview with representative
Other Information

Agriculture
March 11, 2024
Implemented by
Capnia

Agriculture
February 5, 2024
Implemented by
Shift Labs
Agriculture
September 26, 2024
Implemented by
Laerdal Global Health

Agriculture
February 20, 2024
Implemented by
Stone Cold Systems
Agriculture
November 22, 2023
Implemented by
GE (General Electric)

Agriculture
February 6, 2024
Implemented by
Kinnos

Agriculture
March 8, 2024
Implemented by
MTTS

Agriculture
March 8, 2024
Implemented by
Nipon Avionics

Agriculture
January 19, 2024
Implemented by
On the Spot Solutions

Agriculture
March 6, 2024
Implemented by
Project Prana Foundation
Have thoughts on how we can improve?
Give Us Feedback
The protoype models seem to be effective. Further testing should give people confidence in the value of this technology.