
Agriculture
November 22, 2024
AEST Agricultural Waste Charcoal Briquettes
Read SolutionImplemented by
Appropriate Energy Saving Technologies Limited (AEST)
 discontinued
discontinued
Updated on December 19, 2023
·Created on October 21, 2021
PremieBreathe is a breathing aid to help premature newborns in resource-constrained settings.
PremieBreathe is a low-cost breathing aid for newborn babies that provides humidified air flow to neonates and was developed by a start-up formed by a multi-departmental collaboration at Yale University. The product is designed for settings that lack safe respiratory devices and is intended to address neonatal mortality due to respiratory complications. In 2016 PremieBreathe was under trial. We have no further updated information as of 2021.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$350.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
Unknown
Competitive Landscape
Direct competitors include Neovent, FREO2-SIPHON, and Pumani Bubble Continuous Positive Airway Pressure (bCPAP) Device.
Countries
Ethiopia, Malawi, Rwanda
Manufacturing/Building Method
This product is currently in the prototyping phase and not yet manufactured at scale. Prototypes are being developed at Yale University in Prof. Gonzalez's lab.
Intellectural Property Type
Select Type
User Provision Model
The designers have not yet selected their user provision model.
Distributions to Date Status
None - product still in prototype stage
Design Specifications
PremieBreathe, is a humidified high flow nasal cannula (HHFNC) device that delivers variable flows of warmed, humidified, and oxygenated air to provide respiratory support for premature and newborn infants. The device incorporates an ultraviolet water sterilization mechanism to kill waterborne bacteria. It also incorporates reusable, biofouling-resistant tubing rather than the disposable circuit used in the commercial technology, embodied by a mobile, compact housing, designed to incorporate a rechargeable battery source, ensures that our device can provide portable oxygen therapy for critically ill newborns in need of undisrupted support, while in transport between hospitals and health centers.
Technical Support
Provided by the manufacturers
Replacement Components
Replacement components include flow regulators and humidification bottles.
Lifecycle
Unknown
Manufacturer Specified Performance Parameters
The maufacturers set out to develop a low-cost respirator that can safely provide warmed, humidified and blended oxygen to neonates in need.
Vetted Performance Status
Pilot tests were performed in Ethiopia at Ayder Referral Hospital to establish the performance and functional parameters after about a half-a-dozen prototyping iterations.
Safety
The manufacturers have taken steps to test the device in a clinical environment to ensure its safety.
Complementary Technical Systems
Unknown
Academic Research and References
Wang, D., 2016, “PremieBreathe: A Low-Cost Respiratory Aid for the Developing World.”
Matthews, M., et al., 2017, “PREferred Providers,” ASEE Prism, 27(2), pp. 24-31.
2018, “Third WHO global forum on medical devices: international conference center Geneva: Geneva, Switzerland 10–12 May 2017,” No. WHO/EMP/2018.02,World Health Organization.
Compliance with regulations
None
Other Information
None

Agriculture
November 22, 2024
Implemented by
Appropriate Energy Saving Technologies Limited (AEST)

Agriculture
February 5, 2024
Implemented by
Centre for Vision in the Developing World

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 1, 2024
Implemented by
Infantrust Parenting Solutions

Agriculture
February 16, 2024
Implemented by
Equalize Health
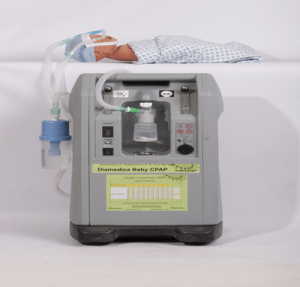
Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
February 29, 2024
Implemented by
Free Wheelchair Mission

Agriculture
December 3, 2024
Implemented by
Greenway

Agriculture
June 22, 2024
Implemented by
Hospitainer
Have thoughts on how we can improve?
Give Us Feedback