
Agriculture
February 5, 2024
Prepex Circumcision Device
Read SolutionImplemented by
Circ MedTech

Updated on February 29, 2024
·Created on August 27, 2015
The ShangRing is a disposable circumcision device.
The ShangRing Circumcision Device is a disposable device for male circumcision that eliminates the need for suturing. Results from Chinese studies have demonstrated that the Shang Ring is both safe and easy to use.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$15.00
Target Users (Target Impact Group)
Small and Medium-sized Enterprises, Public Sector Agencies, NGOs
Distributors / Implementing Organizations
Manufactured by Wuhu Santa Medical Equipment Technology Co. Ltd, distributed to health clinics and hospitals.
Competitive Landscape
Direct competitors include Prepex Circumcision Device.
Regions
Sub-Saharan Africa
Countries
China, Kenya, Uganda, Zambia
Manufacturing/Building Method
The ShangRing is mass produced in China by Wuhu Snnda Medical Treatment Appliance Technology Company Limited.
Intellectural Property Type
Patent
User Provision Model
The device is available at health clinics and hospitals to surgically trained medics. It can also be purchased via online retailer
Distributions to Date Status
600,000 units have been used since 2005. Over 3,000 men have participated in clinical studies in China and Africa between 2007 and 2013.
Age Requirement
Neonates to adults
Infection Rate (%)
<10%
Injection of local anesthesia required (Y/N)
Y
Number of visits per procedure
2
Procedure Time (“skin to skin”)
2-5 minutes
Design Specifications
The ShangRing is a disposable circumcision tool that helps health care providers with limited training to perform circumcision. The device consists of two parts (rings). The outer ring consists of two halves that are hinged together at one end with a ratchet closure. Hemostasis is realized by the pressure applied by the interlocking rings which minimizes bleeding and eliminates the need for sutures. The device must remain on the penis for 5-7 days.
The device is made from plastic, with a weight of 2.5 – 8.5 grams. ShangRign is commercially available in China in 32 sizes, ranging from 9-42 mm in diameter, for use with neonates to adults. The device is pre-sterilized.
Technical Support
The ShangRing must be used by properly surgically trained physicians. Training DVDs, manuals, and curriculum are being developed.
Replacement Components
The device is single-use.
Lifecycle
The device is sterilised by gamma radiation (Cobalt-60 exposure) and has a shelf life of 3 years. It is strictly non-reusable.
Manufacturer Specified Performance Parameters
The manufacturer claims that circumcision with the ShangRing is safer and more acceptable to patients than conventional surgical circumcision methods. Additional performance targets include: • No sutures necessary • Rapid procedural time (2 to 5 min) • Less bleeding • Frenulum is well preserved • Low complication rate • Excellent cosmesis • Easy to teach and learn • High satisfaction among medical providers and patients
Vetted Performance Status
Results from Chinese studies have demonstrated that the Shang Ring is both safe and easy to use. The first report of the Shang Ring in the literature was from a study conducted by Peng et al. who reported low rates of complication among 1,200 Shang Ring circumcisions. Similarly low complication rates were seen in a series of 328 men circumcised with the Shang Ring in another Chinese study by Cheng et al.. A third study by Li et al. conducted in China comparing Shang Ring circumcision with the dorsal slit technique found significantly fewer complications, lower pain levels during and after the procedure, and higher participant satisfaction among participants circumcised with the Shang Ring. Average Shang Ring circumcision times (excluding administration of local anesthesia), were reported in the Chinese studies to be between 3-5 minutes with patient satisfaction rates over 98. Since 2008, a series of studies using the Shang Ring for adult MC have been carried out in Africa according to guidelines established by WHO for clinical evaluation of new devices for adult MC. Results from the studies conducted to date in Africa demonstrate that the Shang Ring has an excellent safety profile, supporting results of Shang Ring use in China and indicating that the Shang Ring could facilitate rapid roll-out of MC for HIV prevention in Africa. The African experience shows that the Shang Ring is easily used by trained non-physicians, which would facilitate task shifting, and is strongly preferred by providers and well-liked by men who have participated in the studies. The vast majority of participants in studies in Africa as well as China have reported few problems and little disruption to daily life while wearing the device. The significantly shorter procedure time for Shang Ring circumcision would allow for many more procedures to be done in a given time frame compared to conventional MC techniques.
Safety
The ShangRing is non-reusable. It should be only used by surgically trained health care providers.
Complementary Technical Systems
None
Academic Research and References
Clinical trials using the Shang Ring device for male circumcision in Africa: a review Mark A. Barone et.al. EngenderHealth, New York, NY, USA; Center for Male Reproductive Medicine and Microsurgery, Department of Urology, Weill Cornell Medical College, New York, NY, USA; EngenderHealth, Nairobi, Kenya Vol 3, No 1 (March 2014). Submitted Dec 02, 2013. Accepted for publication Jan 24, 2014. doi: 10.3978/j.issn.2223-4683.2014.01.09
Clinical application of a new device for minimally invasive circumcision: Yi-Feng Peng et.al. Asian J Androl 2008; 10 (3): 447–454 DOI: 10.1111/j.1745-7262.2008.00411.x
Kigozi G, Musoke R, Watya S, Kighoma N, Ssebbowa P, Serwadda D, Nalugoda F, Makumbi F, Li P, Lee R, et al. The Acceptability and Safety of the Shang Ring for Adult Male Circumcision in Rakai, Uganda. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;63(5):617–621. doi:10.1097/qai.0b013e3182968dda
Sokal DC, Li PS, Zulu R, Awori QD, Combes SL, Simba RO, Lee R, Hart C, Perchal P, Hawry HJ, et al. Randomized Controlled Trial of the Shang Ring Versus Conventional Surgical Techniques for Adult Male Circumcision. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;65(4):447–455. doi:10.1097/qai.0000000000000061
Barone MA, Ndede F, Li PS, Masson P, Awori Q, Okech J, Cherutich P, Muraguri N, Perchal P, Lee R, et al. The Shang Ring Device for Adult Male Circumcision: A Proof of Concept Study in Kenya. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;57(1). doi:10.1097/qai.0b013e3182158967
Feller, Stephen. “Who Pre-Qualifies Shangring Circumcision Device to Curb HIV.” UPI Health, 2015.
Appropedia. “Shangring.” Appropedia: the sustainability wiki. Accessed February 5, 2022.
The Circlist. “The Shangring.” 2020.
Compliance with regulations
The ShangRingTM has obtained FDA (510K) clearance in the US, and CE Mark from the European Union and ISO 13485 certification.
Evaluation methods
Randomized controlled trials tracking user feedback, healing time, blood loss, infection rate and complication rate.
Other Information
Video presentations of the ShangRing™ surgical technique have been presented in various conferences and received awards for excellence.

Agriculture
February 5, 2024
Implemented by
Circ MedTech

Agriculture
February 20, 2024
Implemented by
Intellectual Ventures Lab

Agriculture
March 10, 2024
Implemented by
Disease Diagnostic Group
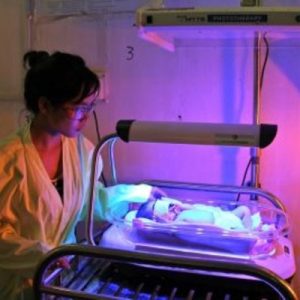
Agriculture
February 8, 2024
Implemented by
Design That Matters (DtM)

Agriculture
January 3, 2024
Implemented by
Stanford Researchers and the International Centre for Diarrhoeal Disease Research, Bangladesh
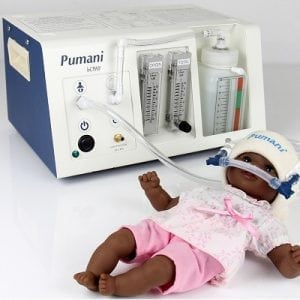
Agriculture
November 24, 2023
Implemented by
Hadleigh Health Technologies, and Rice 360
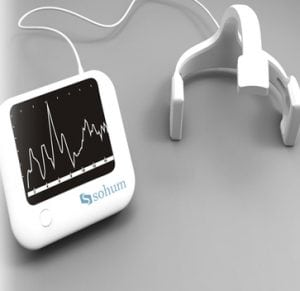
Agriculture
December 12, 2023
Implemented by
Sohum Innovation lab
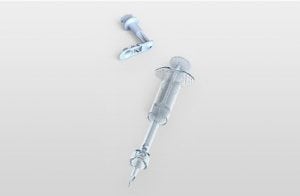
Agriculture
December 19, 2023
Implemented by
Star Syringe

Agriculture
September 26, 2024
Implemented by
Jorge Odón

Agriculture
February 26, 2024
Implemented by
Hemex Health
Have thoughts on how we can improve?
Give Us Feedback