
Agriculture
March 1, 2024
Bilisense Jaundice Treatment Pad
Read SolutionImplemented by
Infantrust Parenting Solutions

Updated on December 12, 2023
·Created on September 8, 2016
SHOEBOX Audiometry is an iPad audiometer application that uses an interactive game to diagnose hearing loss.
SHOEBOX Audiometry is an iPad audiometer application that uses an interactive game to diagnose hearing loss. Calibrated headphones and a web-based data management system are included with the monthly licensing.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises, Public Sector Agencies
Distributors / Implementing Organizations
Clearwater Clinical Limited, Grand Challenges Canada, Business Development Bank of Canada, World Wide Hearing.
Regions
Africa, Central Asia, East Asia, North America, North Asia, South Asia, Southeast Asia
Manufacturing/Building Method
Software development.
Intellectural Property Type
Patent
User Provision Model
The iPad application can be downloaded from the Apple Store. Contact SHOEBOX Audiometry to register for a free 14 day trial.
SHOEBOX Audiometery has partnered with World Wide Hearing's Hearing Express Program to distribute the product. The Hearing Express Program aims to train locals on how to test people’s hearing and provide mobile hearing care kits so that these trained individuals can screen and diagnose hearing loss within their communities. World Wide Hearing works with manufacturers such as Clearwater Clinical to drive down costs associated with of materials. Clearwater charges World Wide Hearing (at a heavily discounted rate) and WWH does not charge for where they donate the unit.
Distributions to Date Status
SHOEBOX Audiometry has over 500 customers in 40 countries Interview with representative
Design Specifications
SHOEBOX requires an iPad, customized software, and calibrated headphones to operate. The ShoeBOX Audiometer creates a Conditioned Play Audiometry (CPA) test where the user plays an interactive listening game that involves touching items on the iPad screen that make sounds. Precise hearing thresholds across all sound frequencies for each ear are recorded as the user plays the game to produce an audiogram. SHOEBOX uses a modified veresion of the gold standard Hughson-Westlake protocol to determine thresholds.The ShoeBOX Audiometry technology automatically adjusts test protocols for masking, ambient noise, and response reliability. The system can generate pure tone or warble tone stimulus and features narrow band noise for masking.
The system is available in three versions, Clinical, diagnostic, and screening. Shoebox Clinical includes additional clinical capabilities required for audiologists and otolaryngologists. Shoebox also features an integrated patient database for adding notes to any patient file and built-in questionnaires to capture patient history. The system includes a HIPAA-compliant web portalthat can be used on any web browser to access, manage, and export hearing test results and patient data.
Additional technical specifications include:
- Device(s) required: iPad Air, iPad Mini, or iPad Pro
- Operating system and version: iOS 8.0 or later
- Languages available: English, Esperanto, French, German, Vietnamese
- Sound generation: 250–8000 Hz, 10–90 dB HL, 0–115 dB HL (Pro edition)
- Accuracy: +/- 1 dB
- Preventative maintenance: annual calibration of headphones
More information can be found here
Technical Support
The manufacturer provides every new user one complimentary hour of “Getting Started” training. Users can receive customized on-site or web-based training for an additional cost. The manufacturer also provides a customer service package that includes annual calibration, a Shoebox Cloud account, ongoing technical support (via email, chat, phone, or web), and software upgrades. The customer service package is free for the first year and is available for an additional charge thereafter. Purchase of the SHOEBOX Audiometer includes a one year warranty. Clearwater provides full technical support and training and the one-year warranty includes a calibration and a new pair of headphones every year. Interview with representative
Calibration of new headphones is offered by ShoeBox. To maintain compliance with ANSI S3.6 standards, ShoeBox offers the following services:
- annual notification of when calibration is due
- automatic swap out of newly calibrated transducers
- return shipment for old transducers
- new calibration certificate for ANSI records management
Replacement Components
N/A
Lifecycle
An iPad's lifespan is approximately 3 years
Manufacturer Specified Performance Parameters
To develop a highly portable and cost-efficient solution to diagnosing hearing loss at a young age.
Vetted Performance Status
A performance study conducted in a remote region in Canada1 and Uganda diagnosed 139 out of 868 children with hearing loss when using the SHOEBOX Audiometer. A study conducted with 49 participants (44 adults, 5 children) indicated that the results from the SHOEBOX Audiometer were within 10 dB of those determined by conventional audiometry for 164 of 172 hearing levels and did not show proportional bias over the testing range.2 Additionally, a clinical study with 85 consecutive patients revealed that there was no statistically significant difference between thresholds obtained by SHOEBOX and traditional audiometry.3
Safety
General iPad safety guidelines can be found here
Complementary Technical Systems
Otoscopes, hearing aids.
Academic Research and References
1.Rourke, R., Kong, D.C., Bromwich, M. Tablet Audiometry in Canada’s North: A Portable and Efficient Method for Hearing Screening.Otolaryngol Head Neck Surg. 2016 Sep; 155(3): 473-8.
2.Thompson, G. P., Sladen, D. P., Hughes Borst, B. J., Still, O. L. Accuracy of a Tablet Audiometer for Measuring Behavioral Hearing Thresholds in a Clinical Population. Otolaryngol Head Neck Surg. 2015 Nov; 153(5): 838-842.
3.Yeung, J., Javidnia, H., Heley, S., Beauregard, Y., Champagne, S., Bromwich, M. The new age of play audiometry: prospective validation testing of an iPad-based play audiometer. J Otolaryngol Head Neck Surg. 2013 Mar; 42(1): 21.
Compliance with regulations
SHOEBOX Audiometry is listed as a Class II device by the FDA and Health Canada. The device conforms to current ANSI, OSHA and CSA requirements. SHOEBOX is both HIPAA & ISO compliant.
Evaluation methods
Clinical validation studies
Other Information
SHOEBOX Audiometry is a recipient of the Grand Challenges Canada award. SHOEBOX Audiometry also won an award for the best technolgoical innovation for care delivery at the Canadian Association of Pediatric Health Centers Innovation Showcase.
Contact ShoeBox for a free trial.

Agriculture
March 1, 2024
Implemented by
Infantrust Parenting Solutions

Agriculture
February 16, 2024
Implemented by
Equalize Health
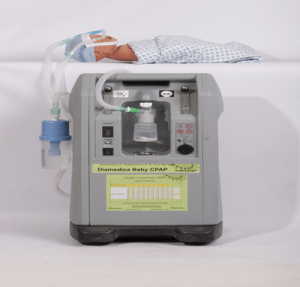
Agriculture
September 27, 2024
Implemented by
Diamedica

Agriculture
December 2, 2024
Implemented by
BioLite

Agriculture
December 3, 2024
Implemented by
Veryday (McKinsey Design)

Agriculture
February 8, 2024
Implemented by
GE Healthcare

Agriculture
January 8, 2024
Implemented by
Healing Waters International

Agriculture
December 1, 2023
Implemented by
International Committee of the Red Cross (ICRC)

Agriculture
December 19, 2023
Implemented by
3D LifePrints

Agriculture
January 3, 2024
Implemented by
Department of Civil and Environmental Engineering, University of Virginia
Have thoughts on how we can improve?
Give Us Feedback