Agriculture
February 5, 2024
Cryo Super Model AA2
Read SolutionImplemented by
Appasamy Associates
Updated on February 5, 2024
·Created on August 2, 2019
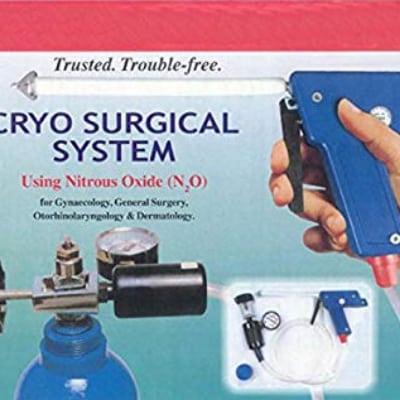
This device is a handheld cryosurgery tool for use in gynecology, protocology, and otorhinolaryngology procedures.
The Super Deluxe Silencer Gun is a cryosurgical tool for use in gynecology, protocology, and otorhinolaryngology procedures. It is hand operated and can be used with probes and hypodermic needles.
Target SDGs
SDG 3: Good Health and Well-Being
Market Suggested Retail Price
$350.00
Target Users (Target Impact Group)
Distributors / Implementing Organizations
Basco India, India Mart
Competitive Landscape
Direct competitors include CryoPen GY2 Cervical Attachment.
Countries
India
Manufacturing/Building Method
The Super Deluxe Silencer Gun is manufactured by Basco India. The body of the device is constructed from ABS plastic.
Intellectural Property Type
Trade Secret
User Provision Model
Users can purchase the Super Deluxe Silencer Gun from distributors including Basco India and India Mart.
Distributions to Date Status
Unknown
Chemicals used
Nitrous Oxide (N2O)
Cooling method
Gas
Clinical applications
Gynecology, protocology, otorhinolaryngology
Consumables
Needles
Power supply type
Battery
Indispensable equipment for function
Needles/probes
Maintenance or calibration required by user at time of use? (Y/N)
Yes
Design Specifications
The Super Deluxe Silencer Gun is made of ABS plastic and high quality hypodermic needles. The probes are specially designed for different types of procedures. The device uses nitrous oxide.
Technical Support
Basco India will repair the device free of cost for one year and will charge ~22 USD (1500 INR)Converted on May 2019 after that year.
Replacement Components
Interchangeable Special Purpose Probes are available for purchase in addition to the main device. Extra washers and a carrying case are also included with the device.
Lifecycle
Unknown
Manufacturer Specified Performance Parameters
Targets include:
Vetted Performance Status
The WHO evaluated this device for gynecological procedures. Researchers from Krishnadevaraya College of Dental Sciences and Hospital in India tested the device in a case of gingival melanin pigmentation and found the results of the procedure to be consistent after 30 months.
Safety
As with all forms of cryosurgery there are certain procedural risks, such as blistering, scarring, or damage to healthy tissue.
Complementary Technical Systems
None
Academic Research and References
Ahmed, S., George, J., Prabhuji, M.L.V. and Lazarus, F., 2017, Cryosurgical Treatment of Gingival Melanin Pigmentation — A 30 Month Follow Up Case Report, Clinical Advances in Periodontics
World Health Organization. “Cryosurgical Equipment for the Treatment of Precancerous Cervical Lesions and Prevention of Cervical Cancer.” WHO Technical Specification, 2012.
Compliance with regulations
Unknown
Evaluation methods
Academic research and partner testing for ability to successfully conduct various medical procedures.

Agriculture
February 5, 2024
Implemented by
Appasamy Associates

Agriculture
February 5, 2024
Implemented by
Appasamy Associates

Agriculture
December 29, 2023
Implemented by
SeaChar

Agriculture
December 10, 2024
Implemented by
Renewit

Agriculture
September 11, 2024
Implemented by
Alvan Blanch

Agriculture
June 25, 2024
Implemented by
Raspberry Pi Foundation

Agriculture
August 21, 2024
Implemented by
Alvan Blanch

Agriculture
June 14, 2024
Implemented by
Global Land Tool Network (GLTN)

Agriculture
June 20, 2024
Implemented by
Electric Vehicle Association of Thailand (EVAT)

Agriculture
March 15, 2024
Have thoughts on how we can improve?
Give Us Feedback