
Agriculture
June 23, 2024
AguaPallet
Read SolutionImplemented by
LoooP Creative Ltd

Updated on June 8, 2024
·Created on October 8, 2018
MEDSINC, is a mobile clinical assessment platform that utilizes proprietary clinical logic to enable anyone anywhere to assess the sickness of a child and provide triage and treatment recommendations.
MEDSINC is a simple-to-use, self-training, mobile, integrated clinical assessment software platform that enables minimally skilled users to effectively assess how sick a child is and provide immediate triage and treatment recommendations. Targeting children two months to five years, MEDSINC guides users through a clinical severity assessment for four critical clinical conditions: respiratory distress, dehydration, infection risk (sepsis), and malnutrition, as well as eight common diagnoses.
Demo video.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Community
Distributors / Implementing Organizations
THINKMD will distribute its product through channel partners, private and public.
Regions
Worldwide
Manufacturing/Building Method
Software development.
Intellectural Property Type
Select Type
User Provision Model
Clients request a demo through the website.
Distributions to Date Status
Thousands of users between clinics and partner NGOs.
Mobile service required
SMS, USSD, Internet
Level of coverage needed
N/A
Device(s) required
Feature phone
Additional features required
None.
Permanent network connectivity required (Y/N)
No: With or without access to cellular/wireless connectivity, the plateform is able to run its services.
Two way communication (Y/N)
Yes.
Usage rate (%)
80% – 95%
Literacy support (Y/N)
Yes.
Languages available
English.
Operating system and version
N/A : web plateforme.
mHealth application
Design Specifications
1) Gather patient demographics;
2) Collect symptoms and illness history;
3) Capture key vital signs and physical findings.
Technical Support
Available.
Replacement Components
N/A
Lifecycle
N/A
Manufacturer Specified Performance Parameters
50 million preventable childhood deaths by 2030.
Vetted Performance Status
MEDSINC platform already delivers 80 to > 95% correlations . For every 1000 MEDSINC users:
Safety
N/A
Complementary Technical Systems
N/A
Academic Research and References
Rodney Nascimento Guimarães, Zilma Reis, May 2017, A multiband reflectance photometric device for reveal gestational age at birth
“Product Demos.” 2021. ThinkMD. July 28, 2021. https://thinkmd.org/product-demos/
“Goal 3.” n.d. Sdgs.Un.Org. Accessed June 7, 2024. https://sdgs.un.org/goals/goal3
User, Guest. 2018. “MEDSINC Pilot Study Closeout Meeting and Certificate Conferment Ceremony —.” EHealth Africa – Building Stronger Health Systems in Africa. July 23, 2018. https://ehealthafrica.squarespace.com/blog/2018/7/23/medsinc-pilot-study-closeout-meeting-and-certificate-conferment-ceremony
“Our Partners.” 2021. ThinkMD. July 28, 2021. https://thinkmd.org/our-partners/
Compliance with regulations
Unknown.
Evaluation methods
MEDSINC has been validated with world-class partners in 5 countries on 4 continents. Accuracy and usability has been confirmed through rigorous user testing and side-by-side clinical trials.
Other Information
None.

Agriculture
June 23, 2024
Implemented by
LoooP Creative Ltd

Agriculture
January 10, 2024
Implemented by
NRSRelief

Agriculture
December 31, 2023
Implemented by
Potential Energy

Agriculture
June 22, 2024
Implemented by
World Bicycle Relief

Agriculture
June 8, 2024
Implemented by
ClickMedix
Agriculture
February 14, 2024
Implemented by
USTAR Biotechnologies (Hangzhou) LTD

Agriculture
January 8, 2024
Implemented by
Gadgil Lab, University of California Berkeley

Agriculture
December 3, 2024
Implemented by
Envirofit International
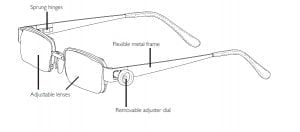
Agriculture
February 5, 2024
Implemented by
EyeJusters

Agriculture
February 8, 2024
Implemented by
GE Healthcare
Have thoughts on how we can improve?
Give Us Feedback