Agriculture
December 19, 2023
Dose Right syringe clips
Read SolutionImplemented by
Hadleigh Health Technologies and Rice University

Updated on December 7, 2023
·Created on August 27, 2015
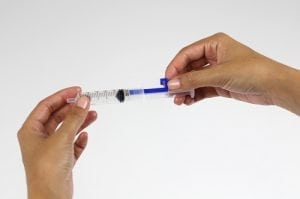
The Uniject™ autodisable injection system is precisely filled with a single drug dose, ensuring that the correct amount of drug is delivered and that none is discarded unnecessarily. It cannot be reused, eliminating the possibility of disease transmission. And it reduces the burden on health systems by combining the vaccine, needle, and syringe into a single unit.
Target SDGs
SDG 3: Good Health and Well-Being
Target Users (Target Impact Group)
Community, Small and Medium-sized Enterprises
Distributors / Implementing Organizations
The Uniject device is licensed to Becton Dickinson. BD manufactures and distributes Uniject to vaccine and pharmaceutical producers which then are given to public health programs. Hepatitis B vaccine and tetanus toxoid are available from manufacturers in India and Indonesia.
Countries
Argentina, Bangladesh, Burkina Faso, Guatemala, Indonesia, Mexico, Niger, Senegal, Uganda
Manufacturing/Building Method
Mass produced in Singapore with a fully automated, high-volume production line.
Intellectural Property Type
Select Type
User Provision Model
Public health programs receive the Uniject pre-filled syrignes from vaccine or pharmaceutical producers The cost of the vaccine or drug may or may not be passed on to the patients depending on the program.
Distributions to Date Status
Over 9 million doses of tetanus toxoid vaccine and 75 million doses of hepatitis B vaccine delivered as of March 2014.
Delivery depths
Subcutaneous & intramuscular
Maximum injection volume (mL)
0.6 mL
Design Specifications
The Uniject is a small bubble of plastic attached to a needle. The small bubble is filled with a single dose of medication. The device measures about 27mm wide and 90mm long. The vaccine reservoir can hold approximately 0.6mL of medication. There is a disc valve to discourage reuse of the device and an isolation barrier to maintain sterility of the vaccine. The needle shield and port maintains needle sterility. The needle can vary based on depth delivery requirements. The optimal size for intramuscular injection is 1 inch long with a 23 gauge.
Technical Support
N/A as this is a single use device. Can be used for self-injections and by those who do not normally give injections.
Replacement Components
N/A. No separate components and not meant to be reused
Lifecycle
Single use device meant to be disposed after one use. Overall biohazard disposal costs should be lower in comparison to traditional syringes because of decreased weight of syringe.
Manufacturer Specified Performance Parameters
Reduces risk of contamination. Maintains sterility of the vaccine and discourages attempts to refill the reservoir. Accurate dose, less buffer stock required and minimizes vaccine wastage. Less overall volume for transport, storage, and disposal.
The device is simple enough that health workers can learn to use it after less than two hours of training. This saves health worker time and can be used by lesser-trained health workers.
Vetted Performance Status
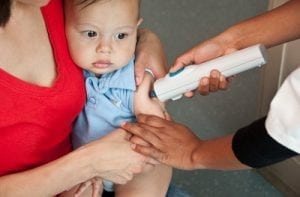
PATH conducted a literature review to identify published and gray literature reporting international experiences with Uniject. This summary synthesizes the experience and evidence on the use of Uniject worldwide with medicines such as the injectable contraceptive Cyclofem®, hepatitis B and tetanus toxoid vaccines, as well as the drug oxytocin, which is used to prevent postpartum hemorrhage. The research findings indicated that Uniject is: -- Easier than a standard syringe: Health providers find Uniject easier to use than a standard syringe, reporting that it is safer, poses less risk of needlestick, and contains the correct dose. For example, midwives in Indonesia who used Uniject to deliver 10,000 hepatitis B vaccines during a ten-month period expressed a preference for the device over a standard syringe. Ninety-nine percent (139 out of 140) of providers in Mali who were surveyed said they preferred Uniject to a standard syringe for administering oxytocin. -- Less painful and intimidating than a standard syringe: Health providers and clients prefer Uniject to a standard syringe as it caused less discomfort. For example, among 30 pregnant women in Bolivia who received injections of tetanus toxoid with Uniject, 50 percent said it was less painful than previous injections received via a conventional syringe, 10 percent reported the pain was comparable to a conventional syringe, and 7 percent said it was more painful. -- Training time is short: Health workers can learn to use Uniject in a short time through peer-led or self guided trainings and materials. -- Safe for self-injection: Health providers say women can safely and correctly give themselves injections with the device. -- Generates less waste: Uniject generates less waste than a traditional needle and syringe. -- Saves time: Use of Uniject requires less time and fewer physical motions by health providers.
Safety
Sharps disposal systems are required as part of use process.
Complementary Technical Systems
Vaccine storage system to maintain appropriate temperatures.
Academic Research and References
1. Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Indijati Soewarso T. Home delivery of heat-stable vaccines in Indonesia: Outreach immunization with a prefilled, single-use injection device. Bulletin of the World Health Organization. 1999;77(2):119–126.
2. Prevention of Postpartum Hemorrhage Initiative. Pilot Use of Oxytocin in a Uniject Device for AMTSL in Mali: Evaluation of the Safety and Feasibility of a New Delivery Technology. Washington, DC:PATH, RTI International, and EngenderHealth; 2008.
3. Tsu VD, Sutanto A, Vaidya K, Coffee P, Widjaya A. Oxytocin in prefilled Uniject injection devices for managing third-stage labor in Indonesia. International Journal of Gynecology & Obstetrics. 2003;83:103–111.
4. Bahamondes L, Marchi NM, Cristofoletti ML et al. Uniject as a delivery system for the once-a-month injectable contraceptive Cyclofem in Brazil. Contraception. 1996;53:115–119.
5. PATH and Viet Nam Ministry of Health. Reducing Postpartum Hemorrhage in Thanh Hoa, Viet Nam: Assessing the Role of Active Management of Third Stage of Labor and of Oxytocin in Ampoules and Uniject Devices. Seattle: PATH; 2005.
6. Quiroga R, Halkyer P, Gil F, Nelson C, Kristensen D. A prefilled injection device for outreach tetanus immunization by Bolivian traditional birth attendants. Pan American Journal of Public Health. 1998;4(1):20–25.
7. Bahamondes L, Marchi N, Nakagava HM et al. Self-administration with Uniject of the once-a-month injecatable contraceptive Cyclofem.
Contraception. 1997;56:301–304
8. PATH. Waste Management Analysis of depo-subQ in Uniject Compared to DMPA Intramuscular Injections. Seattle: PATH; 2010.
9. PATH. Time and motion study with potential human papillomavirus vaccine product presentations. Seattle: PATH; 2009.
10. Tsu, Sutanto, Oxytocin in prefilled Uniject™ injection devices for managing third-stage labor in Indonesia, (requires access)
11. Stewart Otto, At-birth immunisation against hepatitis B using a novel pre-filled immunisation device stored outside the cold chain (requires access)
12. Lloyd J, Technologies for vaccine delivery in the 21st century: A white paper of WHO, UNICEF, USAID and PATH
13. Simosen L, Unsafe injections in the developing world and trasmission of bloodborne pathogens: a review
14. Lloyd Kane, Transmission of hepatitis B, hepatitus C, and human immunodeficiency viruses through unsafe injections in the developing world: model based regional estimates
Compliance with regulations
WHO has pre-qualified both TT and hepatitis B vaccine in the Uniject device.
Evaluation methods
Clinical trials, patient and user feedback
Other Information
Unknown.

Agriculture
December 19, 2023
Implemented by
Hadleigh Health Technologies and Rice University
Agriculture
December 19, 2023
Implemented by
PATH

Agriculture
January 5, 2024
Implemented by
Graftlab

Agriculture
February 6, 2024
Implemented by
SmartPPE

Agriculture
February 6, 2024
Implemented by
Center for Information Technology Research in the Interest of Society and the Banatao Institute

Agriculture
February 21, 2024
Implemented by
SunDanzer

Agriculture
January 11, 2024
Implemented by
Centre for Affordable Water and Sanitation Technologies (CAWST)
Agriculture
June 30, 2024
Implemented by
Biosense
Agriculture
January 10, 2024
Implemented by
Evenproducts

Agriculture
November 3, 2023
Implemented by
Laerdal Global Health
Have thoughts on how we can improve?
Give Us Feedback